A Single Injection of Human Neutralizing Antibody Protects against Zika Virus Infection and Microcephaly in Developing Mouse Embryos
- PMID: 29719255
- PMCID: PMC7104101
- DOI: 10.1016/j.celrep.2018.04.005
A Single Injection of Human Neutralizing Antibody Protects against Zika Virus Infection and Microcephaly in Developing Mouse Embryos
Abstract
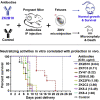
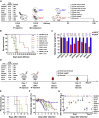
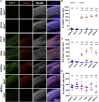
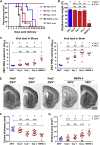
Zika virus (ZIKV) is a mosquito-transmitted flavivirus that is generally benign in humans. However, an emergent strain of ZIKV has become widespread, causing severe pre- and post-natal neurological defects. There is now an urgent need for prophylactic and therapeutic agents. To address this, we investigated six human monoclonal antibodies with ZIKV epitope specificity and neutralizing activity in mouse models of ZIKV infection and microcephaly. A single intraperitoneal injection of these antibodies conveyed distinct levels of adult and in utero protection from ZIKV infection, which closely mirrored their respective in vitro neutralizing activities. One antibody, ZK2B10, showed the most potent neutralization activity, completely protected uninfected mice, and markedly reduced tissue pathology in infected mice. Thus, ZK2B10 is a promising candidate for the development of antibody-based interventions and informs the rational design of ZIKV vaccine.
Keywords: Zika virus; epitope; microcephaly; neural progenitor cells; neutralizing antibody; protection; vaccine.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Patents have been filed for the isolated antibodies, and they are all available for collaboration in research and development through material transfer agreement.
Figures

Similar articles
-
Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus.mBio. 2016 Jul 19;7(4):e01123-16. doi: 10.1128/mBio.01123-16. mBio. 2016. PMID: 27435464 Free PMC article.
-
Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice.Nature. 2016 Dec 15;540(7633):443-447. doi: 10.1038/nature20564. Epub 2016 Nov 7. Nature. 2016. PMID: 27819683 Free PMC article.
-
Perinatal analyses of Zika- and dengue virus-specific neutralizing antibodies: A microcephaly case-control study in an area of high dengue endemicity in Brazil.PLoS Negl Trop Dis. 2019 Mar 11;13(3):e0007246. doi: 10.1371/journal.pntd.0007246. eCollection 2019 Mar. PLoS Negl Trop Dis. 2019. PMID: 30856223 Free PMC article.
-
Zika Virus Infection in Pregnancy, Microcephaly, and Maternal and Fetal Health: What We Think, What We Know, and What We Think We Know.Arch Pathol Lab Med. 2017 Jan;141(1):26-32. doi: 10.5858/arpa.2016-0382-RA. Epub 2016 Sep 16. Arch Pathol Lab Med. 2017. PMID: 27636525 Review.
-
Zika infection and the development of neurological defects.Cell Microbiol. 2017 Jun;19(6). doi: 10.1111/cmi.12744. Epub 2017 May 3. Cell Microbiol. 2017. PMID: 28370966 Review.
Cited by
-
Control of maternal Zika virus infection during pregnancy is associated with lower antibody titers in a macaque model.Front Immunol. 2023 Sep 22;14:1267638. doi: 10.3389/fimmu.2023.1267638. eCollection 2023. Front Immunol. 2023. PMID: 37809089 Free PMC article.
-
ZIKV-envelope proteins induce specific humoral and cellular immunity in distinct mice strains.Sci Rep. 2022 Sep 21;12(1):15733. doi: 10.1038/s41598-022-20183-x. Sci Rep. 2022. PMID: 36131132 Free PMC article.
-
Diagnostic and vaccine potential of Zika virus envelope protein (E) derivates produced in bacterial and insect cells.Front Immunol. 2023 Mar 16;14:1071041. doi: 10.3389/fimmu.2023.1071041. eCollection 2023. Front Immunol. 2023. PMID: 37006270 Free PMC article.
-
Aberrant NAD+ metabolism underlies Zika virus-induced microcephaly.Nat Metab. 2021 Aug;3(8):1109-1124. doi: 10.1038/s42255-021-00437-0. Epub 2021 Aug 12. Nat Metab. 2021. PMID: 34385701
-
Isolation of Monoclonal Antibodies from Zika Virus-Infected Patient Samples.Methods Mol Biol. 2020;2142:261-288. doi: 10.1007/978-1-0716-0581-3_20. Methods Mol Biol. 2020. PMID: 32367373 Free PMC article.
References
-
- Corti D., Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu. Rev. Immunol. 2013;31:705–742. - PubMed
-
- Dejnirattisai W., Wongwiwat W., Supasa S., Zhang X., Dai X., Rouvinski A., Jumnainsong A., Edwards C., Quyen N.T.H., Duangchinda T., et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015;16:170–177. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

