New horizons for multiple sclerosis therapeutics: milestones in the development of ocrelizumab
- PMID: 29719400
- PMCID: PMC5922247
- DOI: 10.2147/NDT.S147874
New horizons for multiple sclerosis therapeutics: milestones in the development of ocrelizumab
Abstract
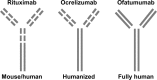
Multiple Sclerosis (MS) is an inflammatory and neurodegenerative disease of the central nervous system, and both T and B cells are involved in its pathogenesis. The vast majority of disease-modifying drugs used for MS act on the inflammatory component of the disease and are approved for use in relapsing-remitting (RR) patients. Ocrelizumab (OCR) is the only MS drug that has been approved by the US Food and Drug Administration (FDA) not only for patients with RRMS but also for patients with primary progressive (PP) MS. OCR is a humanized anti-CD20 monoclonal antibody that can deplete the targeted B cells through antibody-dependent cellular cytotoxicity. Treatment involves administration by intravenous infusion every 6 months. OCR can cause long-lasting B-cell depletion and change the pool of reconstituted B cells. Phase III clinical trials have confirmed the results of previous Phase II studies. In particular, OPERA I and II trials, which were performed in patients with RRMS, showed a reduction in the annualized relapse rate, the risk of disability progression, and the number of new/enlarging T2 lesions and enhancing lesions measured using brain magnetic resonance. The ORATORIO trial, performed in PP subjects, showed that OCR can reduce disability progression, improve performance on the timed 25-foot walk, and decrease the total volume of T2 lesions and the mean number of new or enlarging T2 lesions. The most frequent adverse events were the infusion-related reactions and infections. Infections were mostly nasopharyngitis, as well as upper respiratory and urinary tract infections. OCR gives no indication for severe or opportunistic infections. There is not a clear increased risk of malignancies. Nevertheless, it could not be excluded. Real-life registries will provide more information about the long-term safety, the risk of exposure during pregnancy, and the risk of rare adverse events. In this review, we analyze the evidence regarding the efficacy and the safety of OCR.
Keywords: anti-CD20 antibody; multiple sclerosis; ocrelizumab.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. - PubMed
-
- Brück W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252(Suppl 5):v3–v9. - PubMed
-
- Prineas JW, Connell F. The fine structure of chronically active multiple sclerosis plaques. Neurology. 1978;28(9 Pt 2):68–75. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

