Anti-apoptotic effects of autophagy via ROS regulation in microtubule-targeted and PDGF-stimulated vascular smooth muscle cells
- PMID: 29719457
- PMCID: PMC5928348
- DOI: 10.4196/kjpp.2018.22.3.349
Anti-apoptotic effects of autophagy via ROS regulation in microtubule-targeted and PDGF-stimulated vascular smooth muscle cells
Abstract
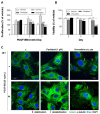
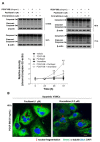
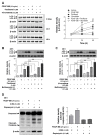
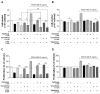
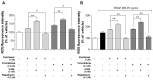
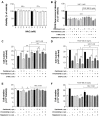
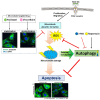
Autophagy has been studied as a therapeutic strategy for cardiovascular diseases. However, insufficient studies have been reported concerning the influence of vascular smooth muscle cells (VSMCs) through autophagy regulation. The aim of the present study was to determine the effects of VSMCs on the regulation of autophagy under in vitro conditions similar to vascular status of the equipped microtubule target agent-eluting stent and increased release of platelet-derived growth factor-BB (PDGF-BB). Cell viability and proliferation were measured using MTT and cell counting assays. Immunofluorescence using an anti-α-tubulin antibody was performed to determine microtubule dynamic formation. Cell apoptosis was measured by cleavage of caspase-3 using western blot analysis, and by nuclear fragmentation using a fluorescence assay. Autophagy activity was assessed by microtubule-associated protein light chain 3-II (LC-II) using western blot analysis. Levels of intracellular reactive oxygen species (ROS) were measured using H2DCFDA. The proliferation and viability of VSMCs were inhibited by microtubule regulation. Additionally, microtubule-regulated and PDGF-BB-stimulated VSMCs increased the cleavage of caspase-3 more than only the microtubule-regulated condition, similar to that of LC3-II, implying autophagy. Inhibitory autophagy of microtubule-regulated and PDGF-BB-stimulated VSMCs resulted in low viability. However, enhancement of autophagy maintained survival through the reduction of ROS. These results suggest that the apoptosis of conditioned VSMCs is decreased by the blocking generation of ROS via the promotion of autophagy, and proliferation is also inhibited. Thus, promoting autophagy as a therapeutic target for vascular restenosis and atherosclerosis may be a good strategy.
Keywords: Apoptosis; Autophagy; Proliferation; Reactive oxygen species; Vascular smooth muscle cell.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
Figures
References
-
- Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf) 2015;214:33–50. - PubMed
-
- van der Wal AC, Becker AE. Atherosclerotic plaque rupture— pathologic basis of plaque stability and instability. Cardiovasc Res. 1999;41:334–344. - PubMed
-
- Ross R. Growth factors in the pathogenesis of atherosclerosis. Acta Med Scand Suppl. 1987;715:33–38. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

