Cold atmospheric plasma as a potential tool for multiple myeloma treatment
- PMID: 29719586
- PMCID: PMC5915053
- DOI: 10.18632/oncotarget.24649
Cold atmospheric plasma as a potential tool for multiple myeloma treatment
Abstract
Multiple myeloma (MM) is a fatal and incurable hematological malignancy thus new therapy need to be developed. Cold atmospheric plasma, a new technology that could generate various active species, could efficiently induce various tumor cells apoptosis. More details about the interaction of plasma and tumor cells need to be addressed before the application of gas plasma in clinical cancer treatment. In this study, we demonstrate that He+O2 plasma could efficiently induce myeloma cell apoptosis through the activation of CD95 and downstream caspase cascades. Extracellular and intracellular reactive oxygen species (ROS) accumulation is essential for CD95-mediated cell apoptosis in response to plasma treatment. Furthermore, p53 is shown to be a key transcription factor in activating CD95 and caspase cascades. More importantly, we demonstrate that CD95 expression is higher in tumor cells than in normal cells in both MM cell lines and MM clinical samples, which suggests that CD95 could be a favorable target for plasma treatment as it could selectively inactivate myeloma tumor cells. Our results illustrate the molecular details of plasma induced myeloma cell apoptosis and it shows that gas plasma could be a potential tool for myeloma therapy in the future.
Keywords: CD95; cold atmospheric plasma; multiple myeloma; reactive oxygen species; selective inactivation.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures

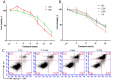
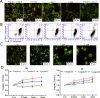





References
-
- Ludwig H, Miguel J, Dimopoulos M, Palumbo A, Sanz RG, Powles R, Lentzsch S, Chen WM, Hou J, Jurczyszyn A. International myeloma working group recommendations for global myeloma care. Leukemia. 2014;28:981–992. - PubMed
-
- Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O, Voorhees P, Leleu X, Johnsen H, Streetly M. Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2016;30:1005–1017. - PubMed
-
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature reviews Drug discovery. 2009;8:579–591. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

