A drug combination targeting hypoxia induced chemoresistance and stemness in glioma cells
- PMID: 29719610
- PMCID: PMC5915077
- DOI: 10.18632/oncotarget.24839
A drug combination targeting hypoxia induced chemoresistance and stemness in glioma cells
Abstract
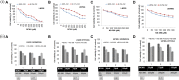
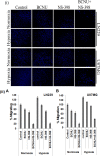
Hypoxia is a characteristic of solid tumors especially Glioblastoma and is critical to chemoresistance. Cancer stem cells present in hypoxic niches are known to be a major cause of the progression, metastasis and relapse. We tried to identify synergistic combinations of drugs effective in both hypoxia and normoxia in tumor cells as well as in cancer stem cells. Since COX-2 is over-expressed in subset of glioblastoma and is also induced in hypoxia, we studied combinations of a prototype Cyclooxygenase (COX-2) inhibitor, NS-398 with various drugs (BCNU, Temozolomide, 2-Deoxy-D-glucose and Cisplatin) for their ability to abrogate chemoresistance under both severe hypoxia (0.2% O2) and normoxia (20% O2) in glioma cells. The only effective combination was of NS-398 and BCNU which showed a synergistic effect in both hypoxia and normoxia. This synergism was evident at sub-lethal doses for either of the single agent. The effectiveness of the combination resulted from increased pro- apoptotic and decreased anti-apoptotic molecules and increased caspase activity. PGE2 levels, a manifestation of COX-2 activity were increased during hypoxia, but were reduced by the combination during both hypoxia and normoxia. The combination reduced the levels of epithelial-mesenchymal transition (EMT) markers. It also resulted in a greater reduction of cell migration. While single drugs could reduce the number of gliomaspheres, the combination successfully abrogated their formation. The combination also resulted in a greater reduction of the cancer stem cell marker CD133. This combination could be a prototype of possible therapy in a tumor with a high degree of hypoxia like glioma.
Keywords: BCNU; COX-2 inhibitor; PGE2; chemosensitization; hypoxia.
Conflict of interest statement
CONFLICTS OF INTEREST The authors disclose no potential conflicts of interest.
Figures







Similar articles
-
Synergistic increase in efficacy of a combination of 2-deoxy-D-glucose and cisplatin in normoxia and hypoxia: switch from autophagy to apoptosis.Tumour Biol. 2016 Sep;37(9):12347-12358. doi: 10.1007/s13277-016-5089-8. Epub 2016 Jun 15. Tumour Biol. 2016. PMID: 27306214
-
Epithelial-to-mesenchymal transition is involved in BCNU resistance in human glioma cells.Neuropathology. 2014 Apr;34(2):128-34. doi: 10.1111/neup.12062. Epub 2013 Sep 22. Neuropathology. 2014. PMID: 24112388
-
HIF-2α mediates a marked increase in migration and stemness characteristics in a subset of glioma cells under hypoxia by activating an Oct-4/Sox-2-Mena (INV) axis.Int J Biochem Cell Biol. 2016 May;74:60-71. doi: 10.1016/j.biocel.2016.02.017. Epub 2016 Feb 26. Int J Biochem Cell Biol. 2016. PMID: 26923292
-
Knockdown of miR-210 decreases hypoxic glioma stem cells stemness and radioresistance.Exp Cell Res. 2014 Aug 1;326(1):22-35. doi: 10.1016/j.yexcr.2014.05.022. Epub 2014 Jun 12. Exp Cell Res. 2014. PMID: 24930954
-
The mechanism between epithelial mesenchymal transition in breast cancer and hypoxia microenvironment.Biomed Pharmacother. 2016 May;80:393-405. doi: 10.1016/j.biopha.2016.02.044. Epub 2016 Apr 17. Biomed Pharmacother. 2016. PMID: 27133080 Review.
Cited by
-
Anti-Glioma Effects of Ligustilide or n-Butylphthalide on Their Own and the Synergistic Effects with Temozolomide via PI3K/Akt Signaling Pathway.Onco Targets Ther. 2023 Nov 22;16:983-994. doi: 10.2147/OTT.S432901. eCollection 2023. Onco Targets Ther. 2023. PMID: 38021448 Free PMC article.
-
Targeting the Epithelial-to-Mesenchymal Transition in Cancer Stem Cells for a Better Clinical Outcome of Glioma.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820948053. doi: 10.1177/1533033820948053. Technol Cancer Res Treat. 2020. PMID: 33089751 Free PMC article. Review.
-
2-Methylthio Conversion of N6-Isopentenyladenosine in Mitochondrial tRNAs by CDK5RAP1 Promotes the Maintenance of Glioma-Initiating Cells.iScience. 2019 Nov 22;21:42-56. doi: 10.1016/j.isci.2019.10.012. Epub 2019 Oct 8. iScience. 2019. PMID: 31654853 Free PMC article.
-
Up-Regulation of Cyclooxygenase-2 (COX-2) Expression by Temozolomide (TMZ) in Human Glioblastoma (GBM) Cell Lines.Int J Mol Sci. 2022 Jan 28;23(3):1545. doi: 10.3390/ijms23031545. Int J Mol Sci. 2022. PMID: 35163465 Free PMC article.
-
New Insights into Mechanisms of Cisplatin Resistance: From Tumor Cell to Microenvironment.Int J Mol Sci. 2019 Aug 24;20(17):4136. doi: 10.3390/ijms20174136. Int J Mol Sci. 2019. PMID: 31450627 Free PMC article. Review.
References
-
- Adamski J, Price A, Dive C, Makin G. Hypoxia-induced cytotoxic drug resistance in osteosarcoma is independent of HIF-1Alpha. PLoS One. 2013;8:e65304. https://doi.org/10.1371/journal.pone.0065304. - DOI - PMC - PubMed
-
- Vinogradov S, Wei X. Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine (Lond) 2012;7:597–615. https://doi.org/10.2217/nnm.12.22. - DOI - PMC - PubMed
-
- Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. https://doi.org/10.1038/nrd4253. - DOI - PMC - PubMed
-
- Safa AR, Saadatzadeh MR, Cohen-Gadol AA, Pollok KE, Bijangi-Vishehsaraei K. Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs. Genes Dis. 2015;2:152–63. https://doi.org/10.1016/j.gendis.2015.02.001. - DOI - PMC - PubMed
-
- Borst P. Cancer drug pan-resistance: pumps, cancer stem cells, quiescence, epithelial to mesenchymal transition, blocked cell death pathways, persisters or what? Open Biol. 2012;2:120066. https://doi.org/10.1098/rsob.120066. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

