Nickel-catalyzed coupling reaction of alkyl halides with aryl Grignard reagents in the presence of 1,3-butadiene: mechanistic studies of four-component coupling and competing cross-coupling reactions
- PMID: 29719693
- PMCID: PMC5903371
- DOI: 10.1039/c7sc04675h
Nickel-catalyzed coupling reaction of alkyl halides with aryl Grignard reagents in the presence of 1,3-butadiene: mechanistic studies of four-component coupling and competing cross-coupling reactions
Abstract
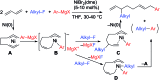
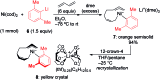
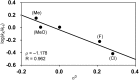
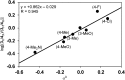
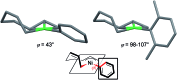
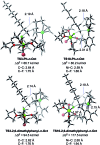
We describe the mechanism, substituent effects, and origins of the selectivity of the nickel-catalyzed four-component coupling reactions of alkyl fluorides, aryl Grignard reagents, and two molecules of 1,3-butadiene that affords a 1,6-octadiene carbon framework bearing alkyl and aryl groups at the 3- and 8-positions, respectively, and the competing cross-coupling reaction. Both the four-component coupling reaction and the cross-coupling reaction are triggered by the formation of anionic nickel complexes, which are generated by the oxidative dimerization of two molecules of 1,3-butadiene on Ni(0) and the subsequent complexation with the aryl Grignard reagents. The C-C bond formation of the alkyl fluorides with the γ-carbon of the anionic nickel complexes leads to the four-component coupling product, whereas the cross-coupling product is yielded via nucleophilic attack of the Ni center toward the alkyl fluorides. These steps are found to be the rate-determining and selectivity-determining steps of the whole catalytic cycle, in which the C-F bond of the alkyl fluorides is activated by the Mg cation rather than a Li or Zn cation. ortho-Substituents of the aryl Grignard reagents suppressed the cross-coupling reaction leading to the selective formation of the four-component products. Such steric effects of the ortho-substituents were clearly demonstrated by crystal structure characterizations of ate complexes and DFT calculations. The electronic effects of the para-substituent of the aryl Grignard reagents on both the selectivity and reaction rates are thoroughly discussed. The present mechanistic study offers new insight into anionic complexes, which are proposed as the key intermediates in catalytic transformations even though detailed mechanisms are not established in many cases, and demonstrates their synthetic utility as promising intermediates for C-C bond forming reactions, providing useful information for developing efficient and straightforward multicomponent reactions.
Figures













Similar articles
-
Cross-coupling reaction of alkyl halides with grignard reagents catalyzed by Ni, Pd, or Cu complexes with pi-carbon ligand(s).Acc Chem Res. 2008 Nov 18;41(11):1545-54. doi: 10.1021/ar800138a. Acc Chem Res. 2008. PMID: 18973349
-
Highly selective biaryl cross-coupling reactions between aryl halides and aryl Grignard reagents: a new catalyst combination of N-heterocyclic carbenes and iron, cobalt, and nickel fluorides.J Am Chem Soc. 2009 Aug 26;131(33):11949-63. doi: 10.1021/ja9039289. J Am Chem Soc. 2009. PMID: 19639999
-
Nickel-Catalyzed Dimerization and Alkylarylation of 1,3-Dienes with Alkyl Fluorides and Aryl Grignard Reagents.Angew Chem Int Ed Engl. 2016 Apr 25;55(18):5550-4. doi: 10.1002/anie.201601126. Epub 2016 Mar 3. Angew Chem Int Ed Engl. 2016. PMID: 26938137
-
Nickel and cobalt-catalyzed coupling of alkyl halides with alkenes via heck reactions and radical conjugate addition.Mini Rev Med Chem. 2013 May 1;13(6):802-13. doi: 10.2174/1389557511313060003. Mini Rev Med Chem. 2013. PMID: 23544460 Review.
-
Ni-Catalyzed C-C Couplings Using Alkyl Electrophiles.Top Curr Chem (Cham). 2016 Oct;374(5):66. doi: 10.1007/s41061-016-0067-6. Epub 2016 Aug 31. Top Curr Chem (Cham). 2016. PMID: 27580894 Review.
Cited by
-
Palladium and Nickel Catalyzed Suzuki Cross-Coupling with Alkyl Fluorides.Org Lett. 2021 Nov 19;23(22):8994-8999. doi: 10.1021/acs.orglett.1c03515. Epub 2021 Nov 1. Org Lett. 2021. PMID: 34723542 Free PMC article.
-
Cryogenic Organometallic Carbon-Fluoride Bond Functionalization with Broad Functional Group Tolerance.J Am Chem Soc. 2025 Feb 19;147(7):5764-5774. doi: 10.1021/jacs.4c13956. Epub 2025 Feb 6. J Am Chem Soc. 2025. PMID: 39912296 Free PMC article.
-
Organocuprate Cross-Coupling Reactions with Alkyl Fluorides.Org Lett. 2022 Dec 2;24(47):8719-8723. doi: 10.1021/acs.orglett.2c03775. Epub 2022 Nov 17. Org Lett. 2022. PMID: 36394939 Free PMC article.
-
General alkyl fluoride functionalization via short-lived carbocation-organozincate ion pairs.Nat Commun. 2024 Feb 29;15(1):1866. doi: 10.1038/s41467-024-45756-4. Nat Commun. 2024. PMID: 38424080 Free PMC article.
-
Formation of Transient Anionic Metal Clusters in Palladium/Diene-Catalyzed Cross-Coupling Reactions.Chemistry. 2019 Oct 17;25(58):13376-13384. doi: 10.1002/chem.201902610. Epub 2019 Sep 17. Chemistry. 2019. PMID: 31335999 Free PMC article.
References
-
-
For reviews:
- Posner G. H. Chem. Rev. 1986;86:831.
- Armstrong R. W., Combs A. P., Tempest P. A., Brown S. D., Keating T. A. Acc. Chem. Res. 1996;29:123.
- Bienaymé H., Hulme C., Oddon G., Schmitt P. Chem.–Eur. J. 2000;6:3321. - PubMed
-
-
-
For reviews:
- Baker R. Chem. Rev. 1973;73:487.
- Keim W. Angew. Chem., Int. Ed. 1990;29:235.
- Sieburth S. M., Cunard N. T. Tetrahedron. 1996;52:6251.
- Lautens M., Klute W., Tam W. Chem. Rev. 1996;96:49. - PubMed
-
-
- Tsuji J. Acc. Chem. Res. 1973;6:8.
- Behr A., Becker M., Beckmann T., Johnen L., Leschinski J., Reyer S. Angew. Chem., Int. Ed. 2009;48:3598. - PubMed
-
- Clement N. D., Routaboul L., Grotevendt A., Jackstell R., Beller M. Chem.–Eur. J. 2008;14:7408. - PubMed
-
- Baker R., Crimmin M. J. J. Chem. Soc., Perkin Trans. 1. 1979:1264.
- Akutagawa S. Bull. Chem. Soc. Jpn. 1976;49:3646.
- Baker R., Nobbs M. S., Winton P. M. J. Organomet. Chem. 1977;137:C43.
- Brun P., Tenaglia A., Waegell B. Tetrahedron. 1985;41:5019.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

