Predictive and mechanistic multivariate linear regression models for reaction development
- PMID: 29719711
- PMCID: PMC5903422
- DOI: 10.1039/c7sc04679k
Predictive and mechanistic multivariate linear regression models for reaction development
Abstract
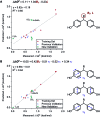
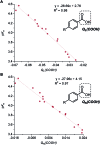
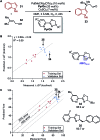
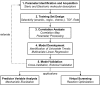
Multivariate Linear Regression (MLR) models utilizing computationally-derived and empirically-derived physical organic molecular descriptors are described in this review. Several reports demonstrating the effectiveness of this methodological approach towards reaction optimization and mechanistic interrogation are discussed. A detailed protocol to access quantitative and predictive MLR models is provided as a guide for model development and parameter analysis.
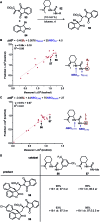
Figures
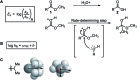
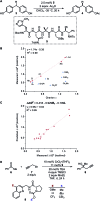

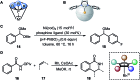
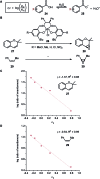








References
-
- Reetz M. T. Angew. Chem., Int. Ed. 2002;41:1335. - PubMed
-
- Carlson R., Design and Optimization in Organic Synthesis, Elsevier, Amsterdam, 1992.
-
- Santanilla A. B., Regalado E. L., Pereira T., Shevlin M., Bateman K., Campeau L.-C., Schneeweis J., Berritt S., Shi Z.-C., Nantermet P., Liu Y., Helmy R., Welch C. J., Vachal P., Davies I. W., Cernak T., Dreher S. D. Science. 2015;347:49. - PubMed
-
- Friedfeld M. R., Shevlin M., Hoyt J. M., Krska S. W., Tudge M. T., Chirik P. J. Science. 2013;342:1076. - PubMed
-
- Collins K. D., Gensch T., Glorius F. Nat. Chem. 2014;6:859. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

