The Physiopathological Role of the Exchangers Belonging to the SLC37 Family
- PMID: 29719821
- PMCID: PMC5913288
- DOI: 10.3389/fchem.2018.00122
The Physiopathological Role of the Exchangers Belonging to the SLC37 Family
Abstract
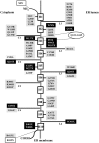
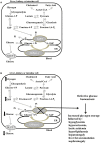
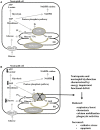
The human SLC37 gene family includes four proteins SLC37A1-4, localized in the endoplasmic reticulum (ER) membrane. They have been grouped into the SLC37 family due to their sequence homology to the bacterial organophosphate/phosphate (Pi) antiporter. SLC37A1-3 are the less characterized isoforms. SLC37A1 and SLC37A2 are Pi-linked glucose-6-phosphate (G6P) antiporters, catalyzing both homologous (Pi/Pi) and heterologous (G6P/Pi) exchanges, whereas SLC37A3 transport properties remain to be clarified. Furthermore, SLC37A1 is highly homologous to the bacterial glycerol 3-phosphate permeases, so it is supposed to transport also glycerol-3-phosphate. The physiological role of SLC37A1-3 is yet to be further investigated. SLC37A1 seems to be required for lipid biosynthesis in cancer cell lines, SLC37A2 has been proposed as a vitamin D and a phospho-progesterone receptor target gene, while mutations in the SLC37A3 gene appear to be associated with congenital hyperinsulinism of infancy. SLC37A4, also known as glucose-6-phosphate translocase (G6PT), transports G6P from the cytoplasm into the ER lumen, working in complex with either glucose-6-phosphatase-α (G6Pase-α) or G6Pase-β to hydrolyze intraluminal G6P to Pi and glucose. G6PT and G6Pase-β are ubiquitously expressed, whereas G6Pase-α is specifically expressed in the liver, kidney and intestine. G6PT/G6Pase-α complex activity regulates fasting blood glucose levels, whereas G6PT/G6Pase-β is required for neutrophil functions. G6PT deficiency is responsible for glycogen storage disease type Ib (GSD-Ib), an autosomal recessive disorder associated with both defective metabolic and myeloid phenotypes. Several kinds of mutations have been identified in the SLC37A4 gene, affecting G6PT function. An increased autoimmunity risk for GSD-Ib patients has also been reported, moreover, SLC37A4 seems to be involved in autophagy.
Keywords: G6PT deficiency; SLC37A1-4; endoplasmic reticulum; glucose-6-phosphate translocase; glycogen storage disease type Ib.
Figures

References
-
- Angaroni C. J., Labrune P., Petit F., Sastre D., Capra A. E., Dodelson de Kremer R., et al. (2006). Glycogen storage disease type Ib without neutropenia generated by a novel splice-site mutation in the glucose-6-phosphate translocase gene. Mol. Genet. Metab. 88, 96–99. 10.1016/j.ymgme.2005.12.011 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

