Urate inhibits microglia activation to protect neurons in an LPS-induced model of Parkinson's disease
- PMID: 29720230
- PMCID: PMC5932803
- DOI: 10.1186/s12974-018-1175-8
Urate inhibits microglia activation to protect neurons in an LPS-induced model of Parkinson's disease
Abstract
Background: Multiple risk factors contribute to the progression of Parkinson's disease, including oxidative stress and neuroinflammation. Epidemiological studies have revealed a link between higher urate level and a lower risk of developing PD. However, the mechanistic basis for this association remains unclear. Urate protects dopaminergic neurons from cell death induced by oxidative stress. Here, we investigated a novel role of urate in microglia activation in a lipopolysaccharide (LPS)-induced PD model.
Methods: We utilized Griess, ELISA, real-time PCR, Western blot, immunohistochemistry, and immunofluorescence to detect the neuroinflammation. For Griess, ELISA, Western blot, and immunofluorescence assay, cells were seeded in 6-well plates pre-coated with poly-L-lysine (PLL) and incubated for 24 h with the indicated drugs. For real-time PCR assay, cells were seeded in 6-well plates pre-coated with PLL and incubated for 6 h with the indicated drugs. For animal experiments, rats were injected with urate or its vehicle twice daily for five consecutive days before and after stereotaxic surgery. Rats were killed and brain tissues were harvested after 4 weeks of LPS injection.
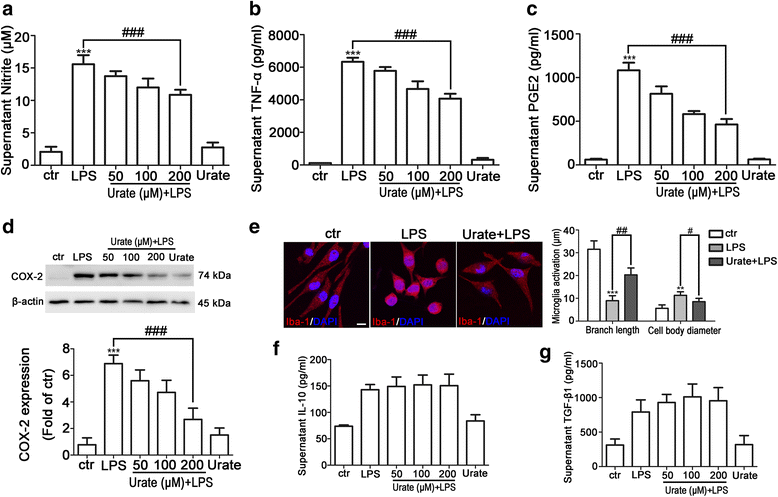
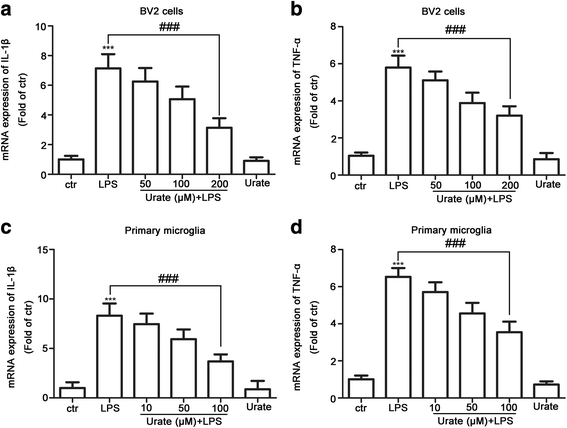
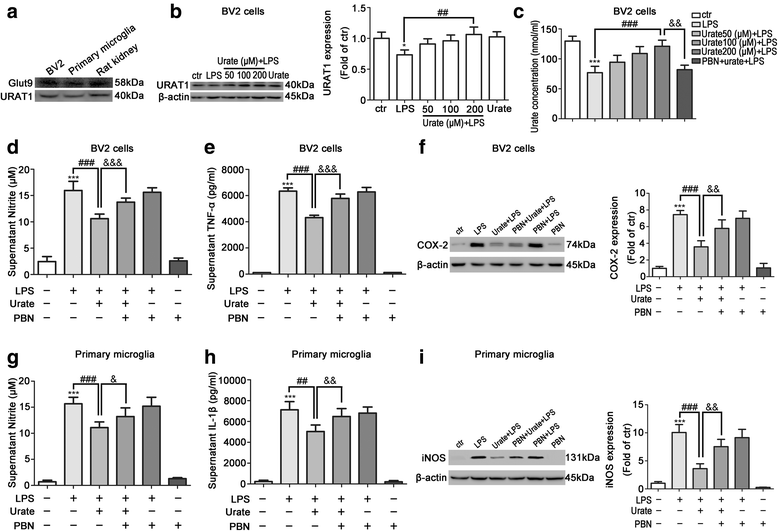
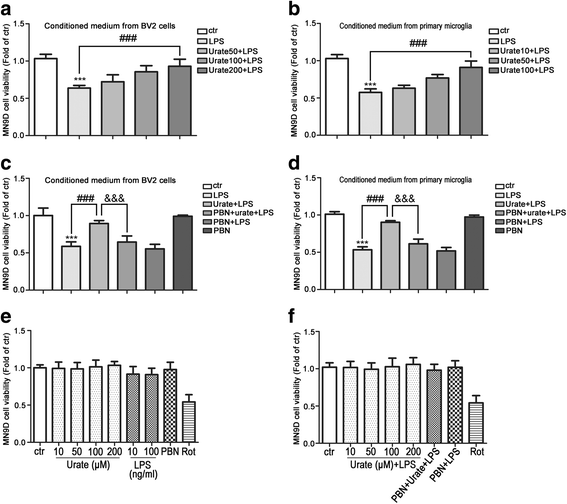
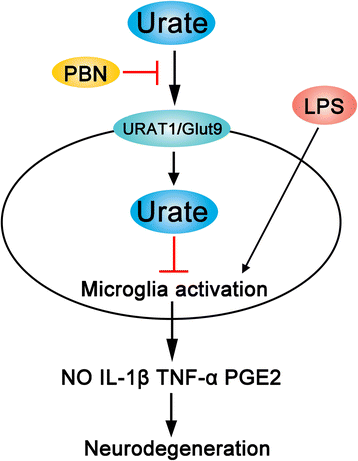
Results: In cultured BV2 cells and rat primary microglia, urate suppressed proinflammatory cytokine production and inducible cyclooxygenase 2 and nitric oxide synthase expression to protect dopaminergic neurons from the toxic effects of activated microglia. The neuroprotective effects of urate may also be associated with the stimulation of anti-inflammatory factors interleukin 10 and transforming growth factor β1. Intracellular urate level was increased in a dose-dependent manner upon co-treatment with urate and LPS as compared with LPS alone, an effect that was abrogated by pretreatment with probenecid (PBN), an inhibitor of both glucose transporter 9 and urate transporter 1 (URAT1). PBN also abolished the anti-inflammatory effect of urate. Consistent with these in vitro observations, the number of tyrosine hydroxylase-positive neurons was decreased and the loss of motor coordination was reversed by urate administration in an LPS-induced rat model of PD. Additionally, increased plasma urate level abolished the reduction of URAT1 expression, the increase in the expression of interleukin-1β, and the number of ionized calcium-binding adaptor molecule 1-positive microglia along with changes in their morphology.
Conclusions: Urate protects neurons against cytotoxicity induced by microglia activation via modulating urate transporter-mediated intracellular urate level.
Keywords: Inflammation; Microglia; Parkinson’s disease; Urate; Urate transporter.
Conflict of interest statement
Ethics approval
All animal experiments were performed in accordance with the guidelines of the Animal Care and Use Committee of Capital Medical University, Beijing, China (2006–0009), and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publications No. 8023, revised 1978).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Transm Supp. 2000;58:143–151. - PubMed
-
- Machado A, Herrera AJ, Venero JL, Santiago M, De PRM, Villarán RF, Espinosa-Oliva AM, Argüelles S, Sarmiento M, Delgado-Cortés MJ. Inflammatory animal model for Parkinson’s disease: the intranigral injection of LPS induced the inflammatory process along with the selective degeneration of nigrostriatal dopaminergic neurons. ISRN Neurol. 2011; 10.5402/2011/476158. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 81372587; 81171886/National Natural Science Foundation of China
- 7132018/Beijing Municipal Natural Science Foundation
- KZ201310025021/Beijing City Board of Education Development Project
- IDHT20140514/Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

