Effects of selexipag and its active metabolite in contrasting the profibrotic myofibroblast activity in cultured scleroderma skin fibroblasts
- PMID: 29720235
- PMCID: PMC5932791
- DOI: 10.1186/s13075-018-1577-0
Effects of selexipag and its active metabolite in contrasting the profibrotic myofibroblast activity in cultured scleroderma skin fibroblasts
Abstract
Background: Myofibroblasts contribute to fibrosis through the overproduction of extracellular matrix (ECM) proteins, primarily type I collagen (COL-1) and fibronectin (FN), a process which is mediated in systemic sclerosis (SSc) by the activation of fibrogenic intracellular signaling transduction molecules, including extracellular signal-regulated kinases 1 and 2 (Erk1/2) and protein kinase B (Akt). Selexipag is a prostacyclin receptor agonist synthesized for the treatment of pulmonary arterial hypertension. The study investigated the possibility for selexipag and its active metabolite (ACT-333679) to downregulate the profibrotic activity in primary cultures of SSc fibroblasts/myofibroblasts and the fibrogenic signaling molecules involved.
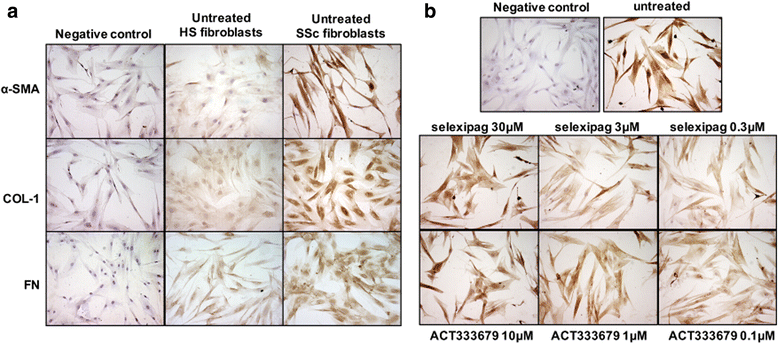
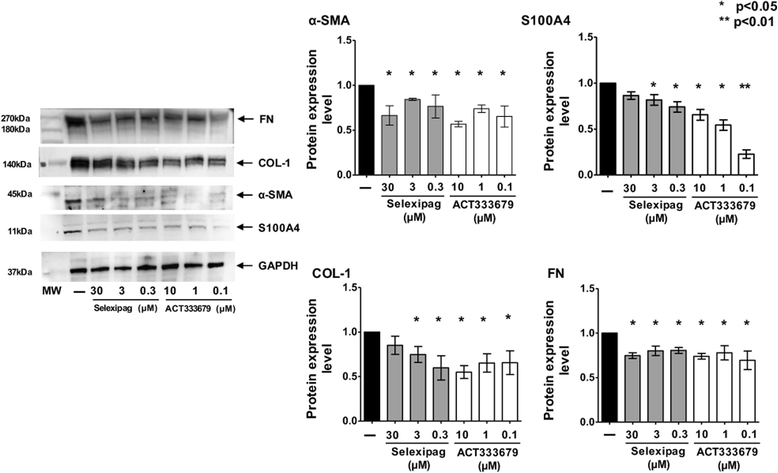
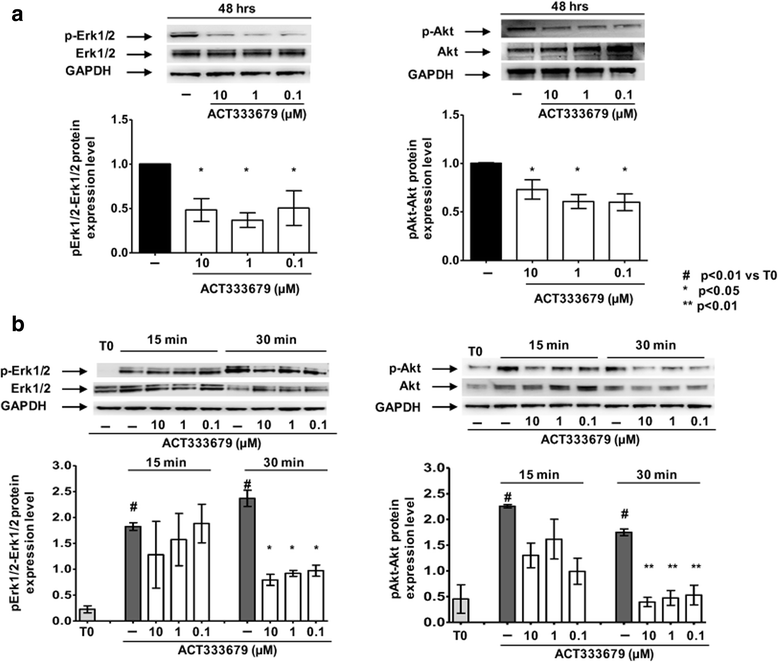
Methods: Fibroblasts from skin biopsies obtained with Ethics Committee (EC) approval from patients with SSc, after giving signed informed consent, were cultured until the 3rd culture passage and then either maintained in normal growth medium (untreated cells) or independently treated with different concentrations of selexipag (from 30 μM to 0.3 μM) or ACT-333679 (from 10 μM to 0.1 μM) for 48 h. Protein and gene expressions of α-smooth muscle actin (α-SMA), fibroblast specific protein-1 (S100A4), COL-1, and FN were investigated by western blotting and quantitative real-time PCR. Erk1/2 and Akt phosphorylation was investigated in untreated and ACT-333679-treated cells by western botting.
Results: Selexipag and ACT-333679 significantly reduced protein synthesis and gene expression of α-SMA, S100A4, and COL-1 in cultured SSc fibroblasts/myofibroblasts compared to untreated cells, whereas FN was significantly downregulated at the protein level. Interestingly, ACT-333679 significantly reduced the phosphorylation of Erk1/2 and Akt in cultured SSc fibroblasts/myofibroblasts.
Conclusions: Selexipag and mainly its active metabolite ACT-333679 were found for the first time to potentially interfere with the profibrotic activity of cultured SSc fibroblasts/myofibroblasts at least in vitro, possibly through the downregulation of fibrogenic Erk1/2 and Akt signaling molecules.
Keywords: Connective tissue diseases; Fibrosis; Prostacyclin receptor agonists; Skin fibroblasts; Systemic sclerosis.
Conflict of interest statement
Authors’ information
MC: full professor in Rheumatology, Director of the Research Laboratory and Academic Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Polyclinic San Martino Hospital; BR: PhD student in Immunology, Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Polyclinic San Martino Hospital; MP: PhD in Internal Medicine and Autoimmunity, Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Polyclinic San Martino Hospital; RB: PhD in Internal Medicine and Autoimmunity, Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Polyclinic San Martino Hospital; ES: trainee in Oncologic Surgery, Department of Surgery, Polyclinic San Martino Hospital; ACT: PhD student in Immunology, Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Polyclinic San Martino Hospital; SSca: Hospital doctor and medical executive in Oncologic Surgery, Department of Surgery, Polyclinic San Martino Hospital; PPT: medical surgeon in Dermatology, Unit of Dermatology, University of Genova, Polyclinic San Martino Hospital AP: full professor of Dermatology, Unit of Dermatology, University of Genova, Polyclinic San Martino Hospital; CC: PhD in Rheumatology, Department of Medicine, Surgery and Neurosciences, Scleroderma Unit, University of Siena; NG: associate professor in Medical Science, Surgery and Neurosciences, Department of Medicine, Surgery and Neurosciences, Scleroderma Unit, University of Siena; SP: assistant professor in Rheumatology, Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Polyclinic San Martino Hospital; CP: assistant professor in Rheumatology, Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Polyclinic San Martino Hospital; AS: associate professor in Rheumatology, Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Polyclinic San Martino Hospital; VS: associate professor in Rheumatology, Department of Rheumatology, Ghent University Hospital; SSol: PhD in Internal Medicine, Autoimmunity and Digestive System Diseases, Division of Clinical Rheumatology, Department of Internal Medicine, University of Genova, Polyclinic San Martino Hospital.
Ethics approval and consent to participate
The study received approval by the local Ethical Board Committee, protocol ID number: 237REG2015.
All patients with SSc and healthy subjects enrolled in the study gave signed informed consent.
Competing interests
MC obtained funds for research from BMS, Actelion, Celgene, and Boehringer. BR, PM, RB, ES, ACT, SSca, PPT, AP CC, NG, SP, CP, AS, VS, and SSol have no competitive interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Ho YY, Lagares D, Tager AM, Kapoor M. Fibrosis—a lethal component of systemic sclerosis. Nature. 2014;10:390–402. - PubMed
-
- Cutolo M, Ruaro B, Smith V. Macrocirculation versus microcirculation and digital ulcers in systemic sclerosis patients: macro-microcirculation and scleroderma. Rheumatology (Oxford). 2017;23 10.1093/rheumatology/kex165. [Epub ahead of print] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

