Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus
- PMID: 29720240
- PMCID: PMC5932888
- DOI: 10.1186/s13075-018-1578-z
Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus
Abstract
Background: Plasmablasts and plasma cells play a key role in many autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). This study was undertaken to evaluate the potential of targeting CD38 as a plasma cell/plasmablast depletion mechanism by daratumumab in the treatment of patients with RA and SLE.
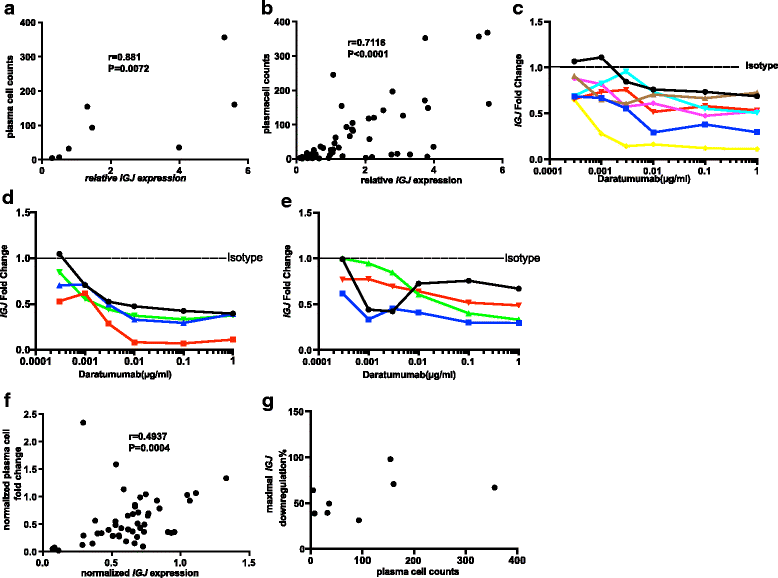
Methods: RNA-sequencing analysis of synovial biopsies from various stages of RA disease progression, flow cytometry analysis of peripheral blood mononuclear cells (PBMC) from patients with RA or SLE and healthy donors, immunohistochemistry assessment (IHC) of synovial biopsies from patients with early RA, and ex vivo immune cell depletion assays using daratumumab (an anti-CD38 monoclonal antibody) were used to assess CD38 as a therapeutic target.
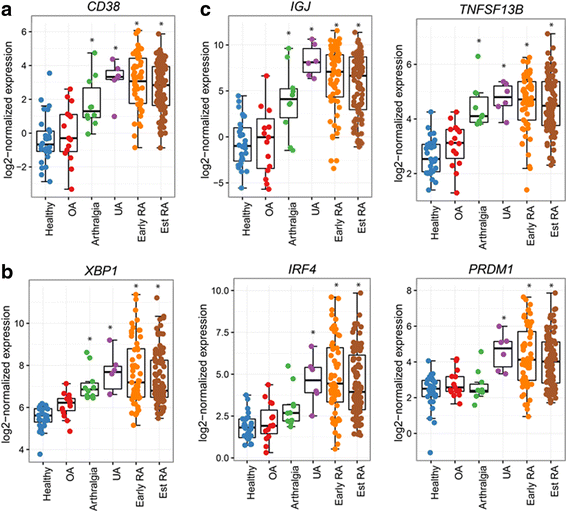
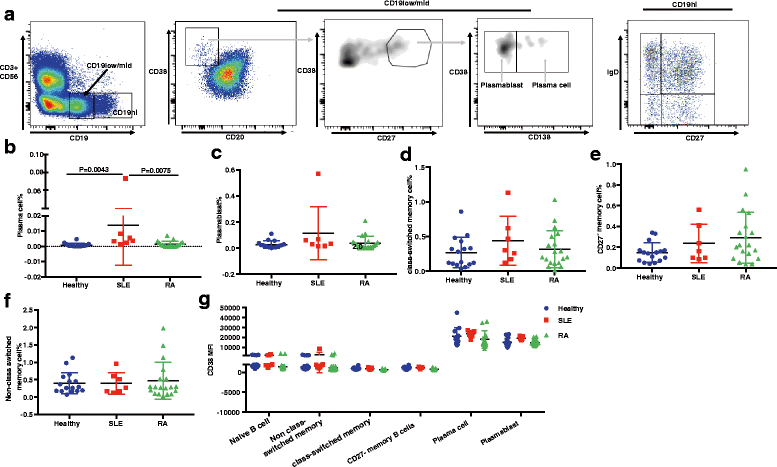
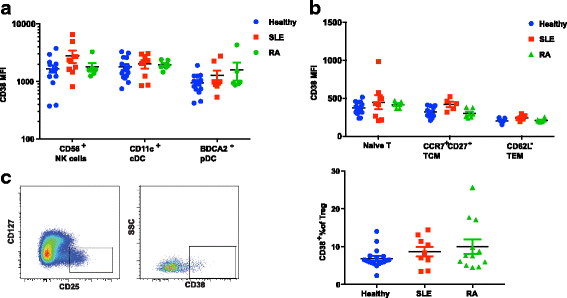
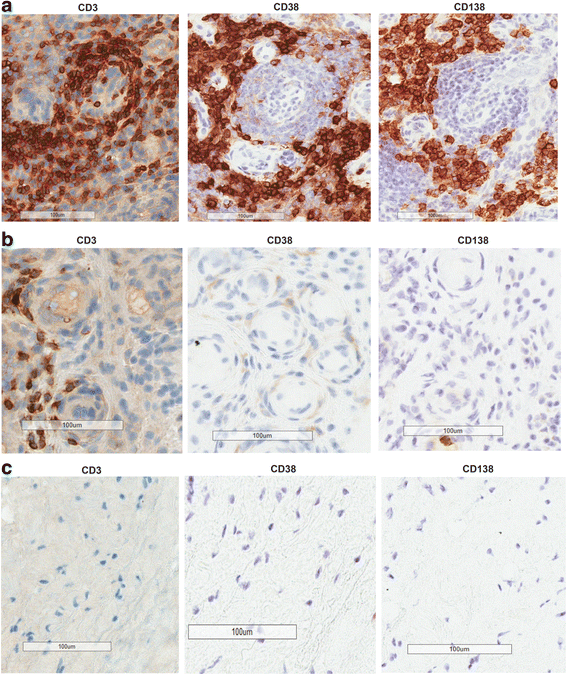
Results: We demonstrated that the plasma cell/plasmablast-related genes CD38, XBP1, IRF4, PRDM1, IGJ and TNFSF13B are significantly up-regulated in synovial biopsies from patients with arthralgia, undifferentiated arthritis (UA), early RA and established RA as compared to healthy controls and control patients with osteoarthritis. In addition, the highest CD38 expression was observed on plasma cells and plasmablasts compared to natural killer (NK) cells, classical dendritic cells (DCs), plasmacytoid DCs (pDCs) and T cells, in blood from healthy controls and patients with SLE and RA. Furthermore, IHC showed CD38 staining in the same region as CD3 and CD138 staining in synovial tissue biopsies from patients with early RA. Most importantly, our data show for the first time that daratumumab effectively depletes plasma cells/plasmablasts in PBMC from patients with SLE and RA in a dose-dependent manner ex vivo.
Conclusion: These results indicate that CD38 may be a potential target for RA disease interception and daratumumab should be evaluated clinically for the treatment of both RA and SLE.
Keywords: CD38; Daratumumab; Plasma cell; Rheumatoid arthritis; Systemic lupus erythematosus.
Conflict of interest statement
Ethics approval and consent to participate
All protocols for collecting synovial biopsies and blood/serum were approved by Institutional Review Board Repatriation General Hospital and Flinders University (Adelaide, Australia), Adelaide Hospital, (Adelaide, Australia), Trinity College Dublin (Dublin, Ireland), St. Vincent’s University Hospital (Dublin, Ireland), and Queen Mary University of London (London, UK). All patients signed the consent form for participating in the study.
Competing interests
SC, AW, XY, JD, MS, CC, HA, AA, IA, LM, SN and YG are current or former employees of Janssen Research & Development, Johnson & Johnson. CP has received research grants and/or honoraria and/or consultation fees from Abbott/AbbVie, Astellas, Astra-Zeneca/MedImmune, BMS, Celgene, Grunenthal, GSK, Janssen/J&J, MSD, Pfizer, Sanofi, Roche/Genentech/Chugai and UCB. MDW, MDS, DJV and UF are recipients of Janssen collaboration research grants. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

