Toward a mechanistic and physiological understanding of a ferredoxin:disulfide reductase from the domains Archaea and Bacteria
- PMID: 29720404
- PMCID: PMC6005431
- DOI: 10.1074/jbc.RA118.002473
Toward a mechanistic and physiological understanding of a ferredoxin:disulfide reductase from the domains Archaea and Bacteria
Abstract
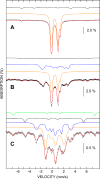
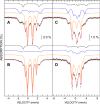
Disulfide reductases reduce other proteins and are critically important for cellular redox signaling and homeostasis. Methanosarcina acetivorans is a methane-producing microbe from the domain Archaea that produces a ferredoxin:disulfide reductase (FDR) for which the crystal structure has been reported, yet its biochemical mechanism and physiological substrates are unknown. FDR and the extensively characterized plant-type ferredoxin:thioredoxin reductase (FTR) belong to a distinct class of disulfide reductases that contain a unique active-site [4Fe-4S] cluster. The results reported here support a mechanism for FDR similar to that reported for FTR with notable exceptions. Unlike FTR, FDR contains a rubredoxin [1Fe-0S] center postulated to mediate electron transfer from ferredoxin to the active-site [4Fe-4S] cluster. UV-visible, EPR, and Mössbauer spectroscopic data indicated that two-electron reduction of the active-site disulfide in FDR involves a one-electron-reduced [4Fe-4S]1+ intermediate previously hypothesized for FTR. Our results support a role for an active-site tyrosine in FDR that occupies the equivalent position of an essential histidine in the active site of FTR. Of note, one of seven Trxs encoded in the genome (Trx5) and methanoredoxin, a glutaredoxin-like enzyme from M. acetivorans, were reduced by FDR, advancing the physiological understanding of FDR's role in the redox metabolism of methanoarchaea. Finally, bioinformatics analyses show that FDR homologs are widespread in diverse microbes from the domain Bacteria.
Keywords: archaea; disulfide; enzyme mechanism; stress; thioredoxin.
© 2018 Prakash et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures







Similar articles
-
Catalytic Activity of the Archetype from Group 4 of the FTR-like Ferredoxin:Thioredoxin Reductase Family Is Regulated by Unique S = 7/2 and S = 1/2 [4Fe-4S] Clusters.Biochemistry. 2024 Jun 18;63(12):1588-1598. doi: 10.1021/acs.biochem.3c00651. Epub 2024 May 31. Biochemistry. 2024. PMID: 38817151 Free PMC article.
-
The function and properties of the iron-sulfur center in spinach ferredoxin: thioredoxin reductase: a new biological role for iron-sulfur clusters.Biochemistry. 1996 Sep 3;35(35):11425-34. doi: 10.1021/bi961007p. Biochemistry. 1996. PMID: 8784198
-
Structural and Biochemical Characterization of a Ferredoxin:Thioredoxin Reductase-like Enzyme from Methanosarcina acetivorans.Biochemistry. 2015 May 19;54(19):3122-8. doi: 10.1021/acs.biochem.5b00137. Biochemistry. 2015. PMID: 25915695
-
Protein disulfides and protein disulfide oxidoreductases in hyperthermophiles.FEBS J. 2006 Sep;273(18):4170-85. doi: 10.1111/j.1742-4658.2006.05421.x. Epub 2006 Aug 23. FEBS J. 2006. PMID: 16930136 Review.
-
Thioredoxins and thioredoxin reductase in chloroplasts: A review.Gene. 2019 Jul 20;706:32-42. doi: 10.1016/j.gene.2019.04.041. Epub 2019 Apr 24. Gene. 2019. PMID: 31028868 Review.
Cited by
-
Functional Prediction and Assignment of Methanobrevibacter ruminantium M1 Operome Using a Combined Bioinformatics Approach.Front Genet. 2020 Dec 16;11:593990. doi: 10.3389/fgene.2020.593990. eCollection 2020. Front Genet. 2020. PMID: 33391347 Free PMC article.
-
Comparative Genomics of the Genus Methanohalophilus, Including a Newly Isolated Strain From Kebrit Deep in the Red Sea.Front Microbiol. 2019 Apr 24;10:839. doi: 10.3389/fmicb.2019.00839. eCollection 2019. Front Microbiol. 2019. PMID: 31068917 Free PMC article.
-
Redox and Thiols in Archaea.Antioxidants (Basel). 2020 May 5;9(5):381. doi: 10.3390/antiox9050381. Antioxidants (Basel). 2020. PMID: 32380716 Free PMC article. Review.
-
A Photosynthesis-Specific Rubredoxin-Like Protein Is Required for Efficient Association of the D1 and D2 Proteins during the Initial Steps of Photosystem II Assembly.Plant Cell. 2019 Sep;31(9):2241-2258. doi: 10.1105/tpc.19.00155. Epub 2019 Jul 18. Plant Cell. 2019. PMID: 31320483 Free PMC article.
-
Crystal Structure of the Apo-Form of NADPH-Dependent Thioredoxin Reductase from a Methane-Producing Archaeon.Antioxidants (Basel). 2018 Nov 17;7(11):166. doi: 10.3390/antiox7110166. Antioxidants (Basel). 2018. PMID: 30453601 Free PMC article.
References
-
- Hanschmann, E. M., Godoy, J. R., Berndt, C., Hudemann, C., and Lillig, C. H. (2013) Thioredoxins, glutaredoxins, and peroxiredoxins. Molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 19, 1539–1605 10.1089/ars.2012.4599 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

