Complex gaze stabilization in mantis shrimp
- PMID: 29720419
- PMCID: PMC5966611
- DOI: 10.1098/rspb.2018.0594
Complex gaze stabilization in mantis shrimp
Erratum in
-
Correction to 'Complex gaze stabilization in mantis shrimp'.Proc Biol Sci. 2018 Jun 27;285(1881):20181244. doi: 10.1098/rspb.2018.1244. Proc Biol Sci. 2018. PMID: 30051856 Free PMC article. No abstract available.
Abstract
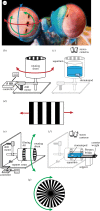
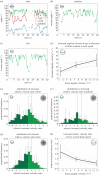
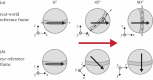
Almost all animals, regardless of the anatomy of the eyes, require some level of gaze stabilization in order to see the world clearly and without blur. For the mantis shrimp, achieving gaze stabilization is unusually challenging as their eyes have an unprecedented scope for movement in all three rotational degrees of freedom: yaw, pitch and torsion. We demonstrate that the species Odontodactylus scyllarus performs stereotypical gaze stabilization in the yaw degree of rotational freedom, which is accompanied by simultaneous changes in the pitch and torsion rotation of the eye. Surprisingly, yaw gaze stabilization performance is unaffected by both the torsional pose and the rate of torsional rotation of the eye. Further to this, we show, for the first time, a lack of a torsional gaze stabilization response in the stomatopod visual system. In the light of these findings, we suggest that the neural wide-field motion detection network in the stomatopod visual system may follow a radially symmetric organization to compensate for the potentially disorientating effects of torsional eye movements, a system likely to be unique to stomatopods.
Keywords: eye movements; gaze stabilization; neural connections; optokinesis; stomatopod.
© 2018 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
-
- Howard J, Dubs A, Payne R. 1984. The dynamics of phototransduction in insects. J. Comp. Physiol. A 154, 707–718. (10.1007/BF01350224) - DOI
-
- Nalbach H-O. 1990. Multisensory control of eyestalk orientation in decapod crustaceans: an ecological approach. J. Crustac. Biol. 10, 382–399. (10.2307/1548328) - DOI
-
- Catz N. 2007. Neuro-opthalmology: neuronal control of eye movements. Basel, Switzerland: Karger.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

