TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy
- PMID: 29720451
- PMCID: PMC5973480
- DOI: 10.1126/scitranslmed.aao4521
TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy
Abstract
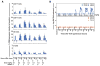
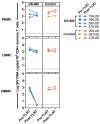
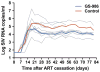
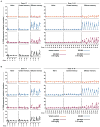
Antiretroviral therapy (ART) can halt HIV-1 replication but fails to target the long-lived latent viral reservoir. Several pharmacological compounds have been evaluated for their ability to reverse HIV-1 latency, but none has demonstrably reduced the latent HIV-1 reservoir or affected viral rebound after the interruption of ART. We evaluated orally administered selective Toll-like receptor 7 (TLR7) agonists GS-986 and GS-9620 for their ability to induce transient viremia in rhesus macaques infected with simian immunodeficiency virus (SIV) and treated with suppressive ART. In an initial dose-escalation study, and a subsequent dose-optimization study, we found that TLR7 agonists activated multiple innate and adaptive immune cell populations in addition to inducing expression of SIV RNA. We also observed TLR7 agonist-induced reductions in SIV DNA and measured inducible virus from treated animals in ex vivo cell cultures. In a second study, after stopping ART, two of nine treated animals remained aviremic for more than 2 years, even after in vivo CD8+ T cell depletion. Moreover, adoptive transfer of cells from aviremic animals could not induce de novo infection in naïve recipient macaques. These findings suggest that TLR7 agonists may facilitate reduction of the viral reservoir in a subset of SIV-infected rhesus macaques.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


References
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. - PubMed
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. - PubMed
-
- Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, Read S, Kallungal B, Palmer S, Medvik K, Lederman MM, Alatrakchi N, Jacobson JM, Wiegand A, Kearney M, Coffin JM, Mellors JW, Eron JJ AIDS Clinical Trials Group A5244 team. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: A randomized controlled trial. PLOS Med. 2010;7:e1000321. - PMC - PubMed
-
- Margolis DM. Mechanisms of HIV latency: An emerging picture of complexity. Curr HIV/AIDS Rep. 2010;7:37–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

