Practical Guidance for Clinical Microbiology Laboratories: Implementing a Quality Management System in the Medical Microbiology Laboratory
- PMID: 29720490
- PMCID: PMC6056841
- DOI: 10.1128/CMR.00062-17
Practical Guidance for Clinical Microbiology Laboratories: Implementing a Quality Management System in the Medical Microbiology Laboratory
Abstract
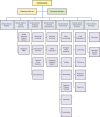
This document outlines a comprehensive practical approach to a laboratory quality management system (QMS) by describing how to operationalize the management and technical requirements described in the ISO 15189 international standard. It provides a crosswalk of the ISO requirements for quality and competence for medical laboratories to the 12 quality system essentials delineated by the Clinical and Laboratory Standards Institute. The quality principles are organized under three main categories: quality infrastructure, laboratory operations, and quality assurance and continual improvement. The roles and responsibilities to establish and sustain a QMS are outlined for microbiology laboratory staff, laboratory management personnel, and the institution's leadership. Examples and forms are included to assist in the real-world implementation of this system and to allow the adaptation of the system for each laboratory's unique environment. Errors and nonconforming events are acknowledged and embraced as an opportunity to improve the quality of the laboratory, a culture shift from blaming individuals. An effective QMS encourages "systems thinking" by providing a process to think globally of the effects of any type of change. Ultimately, a successful QMS is achieved when its principles are adopted as part of daily practice throughout the total testing process continuum.
Keywords: ISO 15189; ISO standard; QMS; continual improvement; quality indicators; quality management system; quality system essentials.
Copyright © 2018 American Society for Microbiology.
Figures
































References
-
- International Organization for Standardization. 2012. Medical laboratories—requirements for quality and competence, 3rd ed ISO document 15189. International Organization for Standardization, Geneva, Switzerland.
-
- Clinical and Laboratory Standards Institute. 2011. A quality management system model for laboratory services; approved guideline, 4th ed CLSI document QMS01-A4. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Schifman RB, Cembrowski GS, Wolk DM, Brisbois JI. 2014. Quality management, p 421–446. In Garcia LS, Bachner P, Baselski VS, Lewis MR, Linscott AJ, Schwab DA, Steele JCH Jr, Weissfeld AS, Wilkinson DS, Wolk DM (ed), Clinical laboratory management, 2nd ed ASM Press, Washington, DC.
-
- US Department of Health and Human Services, Centers for Medicare and Medicaid Services. 2003. Medicare, Medicaid and CLIA programs; laboratory requirements relating to quality systems and certain personnel qualifications. Final rule. 42 CFR 493. Fed Regist 68:3640–3714. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

