Hepatitis C Virus Screening Among Children Exposed During Pregnancy
- PMID: 29720535
- PMCID: PMC5984711
- DOI: 10.1542/peds.2017-3273
Hepatitis C Virus Screening Among Children Exposed During Pregnancy
Abstract
Objectives: Because of the opioid epidemic, hepatitis C virus (HCV) infection is increasing among pregnant women, resulting in an increased risk of perinatal transmission and HCV infection among children. Our primary objectives in this study were to determine the prevalence of HCV among pregnant women and the frequency of pediatric HCV screening.
Methods: A population-based, retrospective cohort of pregnant women who delivered between 2006 and 2014 was identified and classified as HCV infected or HCV uninfected by billing codes. Infant records linked to the HCV-infected pregnant women were identified and queried for HCV tests and the receipt of well-child services, which were defined as the presence of hemoglobin and/or lead testing at or after 9 months of age.
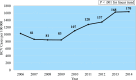
Results: Between 2006 and 2014, 1043 (1.2%) HCV-infected pregnant women delivered, and the HCV prevalence increased by 60%. HCV-infected women were more likely to be <30 years of age (67% vs 53%; P < .001), white (93% vs 72%; P < .001), insured by Medicaid (77% vs 29%; P < .001), and have opiate use disorder (68% vs 1%; P < .001) than HCV-uninfected women. Infants born to HCV-infected women were more likely to be preterm (<37 weeks' gestation; 22% vs 10%; P < .001) and of low birth weight (<2500 g; 23% vs 8%; P < .001). Among 1025 HCV-exposed infants with available pediatric records, 323 (31%) received well-child services, and among these, only 96 (30%) were screened for HCV.
Conclusions: Despite the increased HCV prevalence among pregnant women and the risk of perinatal HCV transmission, HCV-exposed infants are not adequately screened, and many pediatric HCV infections remain undetected.
Copyright © 2018 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Drs Chappell, Bogen, and Krans currently receive research funding from Gilead Sciences related to hepatitis C virus treatment of pregnant and postpartum women. Drs Krans and Chappell receive research funding through Magee-Womens Research Institute from Merck, and Dr Hillier has served as a consultant for Merck. Mr Crowe and Dr Meyn have indicated they have no potential conflicts of interest to disclose.
Figures
References
-
- Suryaprasad AG, White JZ, Xu F, et al. . Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59(10):1411–1419 - PubMed
-
- Zibbell JE, Iqbal K, Patel RC, et al. ; Centers for Disease Control and Prevention . Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458 - PMC - PubMed
-
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical