First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis
- PMID: 29720571
- PMCID: PMC6012572
- DOI: 10.1172/jci.insight.120608
First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis
Abstract
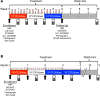
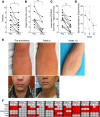
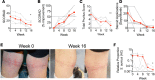
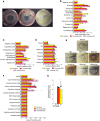
The underlying pathology of atopic dermatitis (AD) includes impaired skin barrier function, susceptibility to Staphylococcus aureus skin infection, immune dysregulation, and cutaneous dysbiosis. Our recent investigation into the potential role of Gram-negative skin bacteria in AD revealed that isolates of one particular commensal, Roseomonas mucosa, collected from healthy volunteers (HVs) improved outcomes in mouse and cell culture models of AD. In contrast, isolates of R. mucosa from patients with AD worsened outcomes in these models. These preclinical results suggested that interventions targeting the microbiome could provide therapeutic benefit for patients with AD. As a first test of this hypothesis in humans, 10 adult and 5 pediatric patients were enrolled in an open-label phase I/II safety and activity trial (the Beginning Assessment of Cutaneous Treatment Efficacy for Roseomonas in Atopic Dermatitis trial; BACTERiAD I/II). Treatment with R. mucosa was associated with significant decreases in measures of disease severity, topical steroid requirement, and S. aureus burden. There were no adverse events or treatment complications. We additionally evaluated differentiating bacterial metabolites and topical exposures that may contribute to the skin dysbiosis associated with AD and/or influence future microbiome-based treatments. These early results support continued evaluation of R. mucosa therapy with a placebo-controlled trial.
Keywords: Allergy; Dermatology; Immunology; Skin.
Conflict of interest statement
Figures
References
-
- Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: A comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49–S57. - PubMed
-
- Latvala J, von Hertzen L, Lindholm H, Haahtela T. Trends in prevalence of asthma and allergy in Finnish young men: nationwide study, 1966-2003. BMJ. 2005;330(7501):1186–1187. doi: 10.1136/bmj.38448.603924.AE. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

