Neutrophils are essential for induction of vaccine-like effects by antiviral monoclonal antibody immunotherapies
- PMID: 29720574
- PMCID: PMC6012508
- DOI: 10.1172/jci.insight.97339
Neutrophils are essential for induction of vaccine-like effects by antiviral monoclonal antibody immunotherapies
Abstract
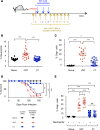
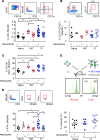
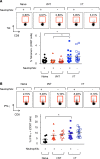
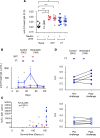
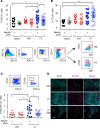
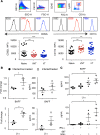
Using a mouse retroviral model, we have shown that mAb-based immunotherapy can induce life-long endogenous protective immunity (vaccine-like effects). This observation has potentially important consequences for treating life-threatening human viral infections. Here, we investigated the role of neutrophils in this effect. Neutrophils are innate immunity effector cells with well-established microbe-killing activities that are rapidly mobilized upon infection. They are also emerging as orchestrators of innate and adaptive immunities. However, their immunomodulatory activity during antiviral mAb immunotherapies has never been studied. Our data reveal that neutrophils have an essential role in immunotherapy-induced immune protection of infected mice. Unexpectedly, neutrophils have a limited effect in controlling viral propagation upon passive immunotherapy administration, which is mostly mediated by NK cells. Instead, neutrophils operate as essential inducers of a potent host humoral antiviral response. Thus, neutrophils play an unexpected key role in protective immunity induction by antiviral mAbs. Our work opens approaches to improve antiviral immunotherapies, as it suggests that preserving neutrophil functions and counts might be required for achieving mAb-induced protective immunity.
Keywords: Adaptive immunity; Immunotherapy; Infectious disease; Neutrophils; Therapeutics.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

