Male-killing toxin in a bacterial symbiont of Drosophila
- PMID: 29720654
- PMCID: PMC5969570
- DOI: 10.1038/s41586-018-0086-2
Male-killing toxin in a bacterial symbiont of Drosophila
Abstract
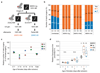
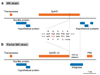
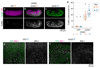
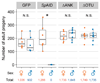
Several lineages of symbiotic bacteria in insects selfishly manipulate host reproduction to spread in a population 1 , often by distorting host sex ratios. Spiroplasma poulsonii2,3 is a helical and motile, Gram-positive symbiotic bacterium that resides in a wide range of Drosophila species 4 . A notable feature of S. poulsonii is male killing, whereby the sons of infected female hosts are selectively killed during development1,2. Although male killing caused by S. poulsonii has been studied since the 1950s, its underlying mechanism is unknown. Here we identify an S. poulsonii protein, designated Spaid, whose expression induces male killing. Overexpression of Spaid in D. melanogaster kills males but not females, and induces massive apoptosis and neural defects, recapitulating the pathology observed in S. poulsonii-infected male embryos5-11. Our data suggest that Spaid targets the dosage compensation machinery on the male X chromosome to mediate its effects. Spaid contains ankyrin repeats and a deubiquitinase domain, which are required for its subcellular localization and activity. Moreover, we found a laboratory mutant strain of S. poulsonii with reduced male-killing ability and a large deletion in the spaid locus. Our study has uncovered a bacterial protein that affects host cellular machinery in a sex-specific way, which is likely to be the long-searched-for factor responsible for S. poulsonii-induced male killing.
Conflict of interest statement
The authors declare no competing financial or non-financial interests.
Figures






Comment in
-
Microbial Misandry: Discovery of a Spiroplasma Male-Killing Toxin.Cell Host Microbe. 2018 Jun 13;23(6):689-690. doi: 10.1016/j.chom.2018.05.011. Cell Host Microbe. 2018. PMID: 29902429
References
-
- Williamson DL, Poulson DF. Sex Ratio Organisms (Spiroplasmas) of Drosophila. In: Whitcomb RF, Tully JG, editors. The Mycoplasmas, Volume III: Plant and Insect Mycoplasmas. Ch. 6. Academic Press; 1979. pp. 175–208.
-
- Williamson DL, et al. Spiroplasma poulsoniisp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int J Syst Bacteriol. 1999;49:611–618. - PubMed
-
- Haselkorn TS. The Spiroplasma heritable bacterial endosymbiont of Drosophila. Fly (Austin) 2010;4:80–87. - PubMed
-
- Tsuchiyama-Omura S, Sakaguchi B, Koga K, Poulson DF. Morphological features of embryogenesis in Drosophila melanogaster infected with a male-killing Spiroplasma. Zoolog Sci. 1988;5:375–383.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

