FTY720 Decreases Tumorigenesis in Group 3 Medulloblastoma Patient-Derived Xenografts
- PMID: 29720672
- PMCID: PMC5932040
- DOI: 10.1038/s41598-018-25263-5
FTY720 Decreases Tumorigenesis in Group 3 Medulloblastoma Patient-Derived Xenografts
Abstract
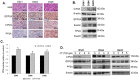
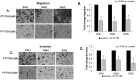
Group 3 tumors account for 28% of medulloblastomas and have the worst prognosis. FTY720, an immunosuppressant currently approved for treatment of multiple sclerosis, has shown antitumor effects in several human cancer cell lines. We hypothesized that treatment with FTY720 (fingolimod) would decrease tumorigenicity in medulloblastoma patient-derived xenografts (PDXs). Three Group 3 medulloblastoma PDXs (D341, D384 and D425) were utilized. Expression of PP2A and its endogenous inhibitors I2PP2A and CIP2A was detected by immunohistochemistry and immunoblotting. PP2A activation was measured via phosphatase activation kit. Cell viability, proliferation, migration and invasion assays were performed after treatment with FTY720. Cell cycle analysis was completed using flow cytometry. A flank model using D425 human medulloblastoma PDX cells was used to assess the in vivo effects of FTY720. FTY720 activated PP2A and led to decreased medulloblastoma PDX cell viability, proliferation, migration and invasion and G1 cell cycle arrest in all three PDXs. FTY720 treatment of mice bearing D425 medulloblastoma PDX tumors resulted in a significant decrease in tumor growth compared to vehicle treated animals. FTY720 decreased viability, proliferation, and motility in Group 3 medulloblastoma PDX cells and significantly decreased tumor growth in vivo. These results suggest that FTY720 should be investigated further as a potential therapeutic agent for medulloblastoma.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

