Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus
- PMID: 29720714
- PMCID: PMC5932050
- DOI: 10.1038/s41598-018-25236-8
Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus
Abstract
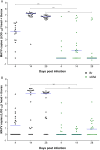
Wolbachia, an intracellular endosymbiont present in up to 70% of all insect species, has been suggested as a sustainable strategy for the control of arboviruses such as Dengue, Zika and Chikungunya. As Mayaro virus outbreaks have also been reported in Latin American countries, the objective of this study was to evaluate the vector competence of Brazilian field-collected Ae. aegypti and the impact of Wolbachia (wMel strain) upon this virus. Our in vitro studies with Aag2 cells showed that Mayaro virus can rapidly multiply, whereas in wMel-infected Aag2 cells, viral growth was significantly impaired. In addition, C6/36 cells seem to have alterations when infected by Mayaro virus. In vivo experiments showed that field-collected Ae. aegypti mosquitoes are highly permissive to Mayaro virus infection, and high viral prevalence was observed in the saliva. On the other hand, Wolbachia-harboring mosquitoes showed significantly impaired capability to transmit Mayaro virus. Our results suggest that the use of Wolbachia-harboring mosquitoes may represent an effective mechanism for the reduction of Mayaro virus transmission throughout Latin America.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Lourenço de Oliveira, R. Biologia e comportamento do vetor. in DengueTeorias e práticas (ed. Valle, D.)75–92 (2015).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

