Phase 1/2 trial of GCLAM with dose-escalated mitoxantrone for newly diagnosed AML or other high-grade myeloid neoplasms
- PMID: 29720734
- PMCID: PMC6192860
- DOI: 10.1038/s41375-018-0135-8
Phase 1/2 trial of GCLAM with dose-escalated mitoxantrone for newly diagnosed AML or other high-grade myeloid neoplasms
Abstract
Outcomes with "7 + 3" are often unsatisfactory in acute myeloid leukemia (AML). Trials demonstrating improved outcomes with high-dose cytarabine, addition of cladribine, or escalated anthracycline doses prompted a phase 1/2 study (NCT02044796) of G-CSF, cladribine, high-dose cytarabine, and dose-escalated mitoxantrone (GCLAM) in adults with newly-diagnosed AML or other high-grade myeloid neoplasms. One hundred and twenty-one patients, median age 60 (range 21-81) years, were enrolled. In phase 1, cohorts of 6-12 patients were assigned to 12-18 mg/m2/day of mitoxantrone as part of GCLAM. Because all dose levels were well-tolerated, mitoxantrone at 18 mg/m2 was declared the recommended phase 2 dose (RP2D). 74/94 (79%) patients treated at the RP2D achieved a complete remission (CR; 67/74 without measureable residual disease [MRD]) for an overall MRDneg CR rate of 71% (primary phase 2 endpoint). Seven patients achieved a CR with incomplete blood count recovery (CRi; 7%, 5 MRDneg) for a CR/CRi rate of 81/94 (86%). Four-week mortality was 2%. After adjustment, the MRDneg CR and CR/CRi rates compared favorably to 100 matched controls treated with 7 + 3 at our center and 245 matched patients treated with 7 + 3 on a cooperative group trial. Our data indicate GCLAM with mitoxantrone at 18 mg/m2/day is safe and induces high-quality remissions in adults with newly-diagnosed AML.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

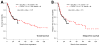
References
-
- Yates JW, Wallace HJ, Jr, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57(4):485–488. - PubMed
-
- Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381(9865):484–495. - PubMed
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. - PubMed
-
- Godwin CD, Gale RP, Walter RB. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia. 2017;31(9):1855–1868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

