Use of Individual Pharmacokinetics to Improve Time to Therapeutic Vancomycin Trough in Pediatric Oncology Patients
- PMID: 29720909
- PMCID: PMC5916451
- DOI: 10.5863/1551-6776-23.2.92
Use of Individual Pharmacokinetics to Improve Time to Therapeutic Vancomycin Trough in Pediatric Oncology Patients
Abstract
Objective: Optimization of vancomycin dosing is difficult in children, given rapid drug clearance and patient heterogeneity. We sought to evaluate the impact of dosing using individual pharmacokinetic parameters on time to goal trough concentration in pediatric oncology patients.
Methods: A retrospective review was conducted to assess vancomycin dosing in the pediatric oncology unit at Loma Linda University Children's Hospital between January 2013 and August 2013 (standard dosing group [SDG]). These patients were compared to those in a prospective arm that used pharmacokinetic dosing (pharmacokinetic dosing group [PKG]) between March 2014 and May 2015. Outcomes included percent of patients reaching a target trough by the specified time points, number of dose adjustments, number of serum concentrations drawn, and number of patients with supratherapeutic troughs.
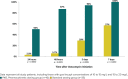
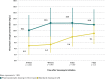
Results: Of 35 patients meeting inclusion criteria for the SDG, 2 (5.7%) reached goal trough concentration by 48 hours, compared with 14 of 16 patients (87%) in the PKG (p = 0.0001). Significantly more patients reached their goal trough at each time point in the PKG. There was no difference in number of dose adjustments, but significantly more concentrations were drawn on average in the PKG (mean, 4.6 versus 3.1, p = 0.02). In the SDG and PKG, respectively, 1 patient and 3 patients had supratherapeutic trough concentrations (p = 0.09).
Conclusions: Dosing using individual pharmacokinetic parameters led to a significant reduction in time to attain the desired vancomycin trough concentration in our pediatric oncology patients. Given the wide variation in dose requirements in this and other studies, application of patient-specific pharmacokinetics is essential to optimize vancomycin dosing in pediatric patients.
Keywords: Sawchuk-Zaske; oncology; pediatrics; pharmacokinetics; therapeutic drug monitoring; vancomycin.
Conflict of interest statement
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
Similar articles
-
Empirical Vancomycin Dosing in Pediatric Patients with Congenital Heart Disease and the Impact of Cardiopulmonary Bypass on Trough Concentrations.Pharmacotherapy. 2017 Nov;37(11):1341-1346. doi: 10.1002/phar.2019. Epub 2017 Sep 28. Pharmacotherapy. 2017. PMID: 28833385
-
Evaluation of vancomycin dosing and corresponding drug concentrations in pediatric patients.Hosp Pediatr. 2014 Nov;4(6):342-7. doi: 10.1542/hpeds.2014-0019. Hosp Pediatr. 2014. PMID: 25362075
-
Assessment of vancomycin dosing and subsequent serum concentrations in pediatric patients.Ann Pharmacother. 2011 May;45(5):582-9. doi: 10.1345/aph.1P588. Epub 2011 Apr 26. Ann Pharmacother. 2011. PMID: 21521865
-
The pharmacokinetic/pharmacodynamic rationale for administering vancomycin via continuous infusion.J Clin Pharm Ther. 2015 Jun;40(3):259-65. doi: 10.1111/jcpt.12270. Epub 2015 Apr 11. J Clin Pharm Ther. 2015. PMID: 25865426 Review.
-
Vancomycin Dosing in Obese Patients: Special Considerations and Novel Dosing Strategies.Ann Pharmacother. 2018 Jun;52(6):580-590. doi: 10.1177/1060028017750084. Epub 2017 Dec 21. Ann Pharmacother. 2018. PMID: 29262697 Review.
Cited by
-
Does vancomycin administered at an empirical dose ensure coverage of pediatric patients against gram-positive pathogens?Rev Bras Ter Intensiva. 2020 Jul-Sep;32(3):391-397. doi: 10.5935/0103-507X.20200067. Rev Bras Ter Intensiva. 2020. PMID: 33053028 Free PMC article.
-
Individualized Vancomycin Dosing with Therapeutic Drug Monitoring and Pharmacokinetic Consultation Service: A Large-Scale Retrospective Observational Study.Drug Des Devel Ther. 2021 Mar 4;15:423-440. doi: 10.2147/DDDT.S285488. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33692613 Free PMC article.
-
Optimizing Vancomycin Dosing in Chronic Kidney Disease by Deriving and Implementing a Web-Based Tool Using a Population Pharmacokinetics Analysis.Front Pharmacol. 2019 Jun 11;10:641. doi: 10.3389/fphar.2019.00641. eCollection 2019. Front Pharmacol. 2019. PMID: 31244657 Free PMC article.
-
A multicentric, randomized, controlled clinical trial to study the impact of bedside model-informed precision dosing of vancomycin in critically ill children-BENEFICIAL trial.Trials. 2024 Oct 10;25(1):669. doi: 10.1186/s13063-024-08512-z. Trials. 2024. PMID: 39390583 Free PMC article.
-
Comparison of Vancomycin Trough-Based and 24-Hour Area Under the Curve Over Minimum Inhibitory Concentration (AUC/MIC)-Based Therapeutic Drug Monitoring in Pediatric Patients.J Pediatr Pharmacol Ther. 2023;28(5):430-438. doi: 10.5863/1551-6776-28.5.430. Epub 2023 Oct 3. J Pediatr Pharmacol Ther. 2023. PMID: 38130493 Free PMC article.
References
-
- Eiland LS, English TM, Eiland EH III. . Assessment of vancomycin dosing and subsequent serum concentrations in pediatric patients. Ann Pharmacother. 2011; 45 5: 582– 589. - PubMed
-
- Glover ML, Cole E, Wolfsdorf J.. Vancomycin dosage requirements among pediatric intensive care unit patients with normal renal function. J Crit Care. 2000; 15 1: 1– 4. - PubMed
-
- Heble DE Jr, McPherson C, Nelson MP, . et al. Vancomycin trough concentrations in overweight or obese pediatric patients. Pharmacotherapy. 2013; 33 12: 1273– 1277. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources