A Real-World Data Study to Evaluate Treatment Patterns, Clinical Characteristics and Survival Outcomes for First- and Second-Line Treatment in Locally Advanced and Metastatic Urothelial Cancer Patients in Germany
- PMID: 29721042
- PMCID: PMC5929077
- DOI: 10.7150/jca.23162
A Real-World Data Study to Evaluate Treatment Patterns, Clinical Characteristics and Survival Outcomes for First- and Second-Line Treatment in Locally Advanced and Metastatic Urothelial Cancer Patients in Germany
Abstract
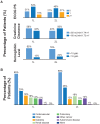
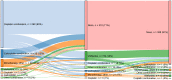
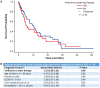
Background: Worldwide, urothelial carcinoma (UC) is a common cause of morbidity and mortality. In particular, the incidence of bladder cancer varies widely across Europe; Germany has the ninth highest international age-standardized incidence. For advanced UC or metastatic UC (mUC), platinum-based combination chemotherapy is the standard first-line (1L) treatment; however, there is wide heterogeneity of second-line (2L) treatments, ranging from vinflunine in parts of Europe to taxanes and other agents elsewhere in Europe, in the United States and globally. Limited data exist on treatment patterns and outcomes in patients with advanced UC or mUC in the routine clinical setting in Germany. The objective of this study was to describe clinical characteristics, treatment patterns and subsequent outcomes in this setting. Methods: This retrospective observational cohort analysis evaluated 1L and 2L treatment patterns and overall survival (OS) in patients aged ≥18 years with advanced UC or mUC (T4b, N2-3 and/or M1) at office-based urology and academic as well as nonacademic urology clinics throughout Germany between 1 November 2009 and 2 June 2016. Data were obtained through the GermanOncology database and additional treatment centers using similar electronic case report forms. Results: Among the 435 patients included in the analysis, 435 received 1L treatment and 125 received 2L treatment. Median age at start of 1L treatment was 69 years, 75% of patients were male, 75% were current or ex-smokers, 15% had hemoglobin <10 g/dL and 44% had creatinine clearance<60 mL/min/1.73; proportions were similar with 2L treatment. Cardiovascular disease was the most frequently reported comorbidity (65%), followed by diabetes (19%). Most patients (77%) received 1L platinum-based combination treatment (most commonly gemcitabine + cisplatin, 83%). Of those treated with 2L treatment, 66% received a single agent (most commonly vinflunine, 71%). Median OS (95% CI) with 1L treatment was 16.1 months (13.7-19.2) overall and 17.7 months (14.4-24.2) with 1L cisplatin + gemcitabine. In the 1L setting, 12-month OS was 61%, 24-month OS was 39% and 36-month OS was 26%. Median (95% CI) OS with 2L treatment was 9.2 months (5.5-11.6) overall and 5.9 months (4.1-12.6) with 2L vinflunine. In the 2L setting, OS rates for the same time periods were 40%, 22% and 8%, respectively. Median (95% CI) progression-free survival was 7 months (6.4-8.1) and 4 months (3.0-4.8), respectively, in the 1L and 2L settings. Objective response rates were 34% in the 1L setting and 14% in the 2L setting. No difference in OS by sex or smoking status was noted. Patients with or without renal impairment had a 12-month OS of 54% or 69%, respectively. OS at 12 months was 63% among patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1 vs 53% among patients with an ECOG PS of ≥2. Cox regression analysis found no difference in OS between vinflunine and other 2L treatments (P = 0.69). Conclusions: This study provides a contemporary multicenter assessment of real-world treatment patterns and outcomes among palliatively treated patients with UC in Germany. The findings were generally consistent with the poor treatment outcomes observed globally, underscoring the need for effective 1L and 2L treatment for advanced UC or mUC.
Keywords: German clinical practice; first-line treatment; metastatic urothelial carcinoma; second-line treatment; treatment patterns.
Conflict of interest statement
Competing Interests: Güenter Niegisch has served in a consulting or advisory role with Roche Pharma AG, has received research funding from 4SC and has received travel and accommodations from Pfizer. Shih-Wen Lin and Julie Pavlova are employees of Genentech and Roche and have stock ownership.
Figures

References
-
- International Agency for Research on Cancer. World Cancer Report 2014. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports....
-
- Burger M, Catto JW, Dalbagni G. et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–241. - PubMed
-
- International Agency for Research on Cancer. Country factsheets: Germany. 2012. http://eco.iarc.fr/eucan/Country.aspx?ISOCountryCd=276.
-
- Cancer in Germany. Cancer in Germany 2011/2012. http://www.krebsdaten.de/Krebs/EN/Content/Publications/Cancer_in_Germany....
LinkOut - more resources
Full Text Sources
Other Literature Sources

