Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice
- PMID: 29721087
- PMCID: PMC5928897
- DOI: 10.7150/thno.23620
Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice
Erratum in
-
Erratum: Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice: Erratum.Theranostics. 2022 Jul 8;12(12):5334. doi: 10.7150/thno.74941. eCollection 2022. Theranostics. 2022. PMID: 35910790 Free PMC article.
Abstract
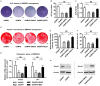
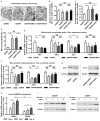
Rational: Senescence of mesenchymal stem cells (MSCs) and the related functional decline of osteogenesis have emerged as the critical pathogenesis of osteoporosis in aging. Resveratrol (RESV), a small molecular compound that safely mimics the effects of dietary restriction, has been well documented to extend lifespan in lower organisms and improve health in aging rodents. However, whether RESV promotes function of senescent stem cells in alleviating age-related phenotypes remains largely unknown. Here, we intend to investigate whether RESV counteracts senescence-associated bone loss via osteogenic improvement of MSCs and the underlying mechanism. Methods: MSCs derived from bone marrow (BMMSCs) and the bone-specific, senescence-accelerated, osteoblastogenesis/osteogenesis-defective mice (the SAMP6 strain) were used as experimental models. In vivo application of RESV was performed at 100 mg/kg intraperitoneally once every other day for 2 months, and in vitro application of RESV was performed at 10 μM. Bone mass, bone formation rates and osteogenic differentiation of BMMSCs were primarily evaluated. Metabolic statuses of BMMSCs and the mitochondrial activity, transcription and morphology were also examined. Mitofilin expression was assessed at both mRNA and protein levels, and short hairpin RNA (shRNA)-based gene knockdown was applied for mechanistic experiments. Results: Chronic intermittent application of RESV enhances bone formation and counteracts accelerated bone loss, with RESV improving osteogenic differentiation of senescent BMMSCs. Furthermore, in rescuing osteogenic decline under BMMSC senescence, RESV restores cellular metabolism through mitochondrial functional recovery via facilitating mitochondrial autonomous gene transcription. Molecularly, in alleviating senescence-associated mitochondrial disorders of BMMSCs, particularly the mitochondrial morphological alterations, RESV upregulates Mitofilin, also known as inner membrane protein of mitochondria (Immt) or Mic60, which is the core component of the mitochondrial contact site and cristae organizing system (MICOS). Moreover, Mitofilin is revealed to be indispensable for mitochondrial homeostasis and osteogenesis of BMMSCs, and that insufficiency of Mitofilin leads to BMMSC senescence and bone loss. More importantly, Mitofilin mediates resveratrol-induced mitochondrial and osteogenic improvements of BMMSCs in senescence. Conclusion: Our findings uncover osteogenic functional improvements of senescent MSCs as critical impacts in anti-osteoporotic practice of RESV, and unravel Mitofilin as a novel mechanism mediating RESV promotion on mitochondrial function in stem cell senescence.
Keywords: Mitofilin/IMMT/Mic60; mesenchymal stem cells; osteogenesis; osteoporosis; resveratrol; senescence-accelerated mice.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Goodell MA, Rando TA. Stem cells and healthy aging. Science. 2015;350:1199–204. - PubMed
-
- Sui BD, Hu CH, Zheng CX, Jin Y. Microenvironmental Views on Mesenchymal Stem Cell Differentiation in Aging. J Dent Res. 2016;95:1333–40. - PubMed
-
- Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16:239–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

