TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade
- PMID: 29721366
- PMCID: PMC5927535
- DOI: 10.1080/2162402X.2017.1412902
TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade
Abstract
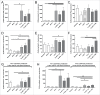
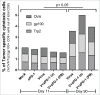
Releasing the patient's immune system against their own malignancy by the use of checkpoint inhibitors is delivering promising results. However, only a subset of patients currently benefit from them. One major limitation of these therapies relates to the inability of T cells to detect or penetrate into the tumor resulting in unresponsiveness to checkpoint inhibition. Virotherapy is an attractive tool for enabling checkpoint inhibitors as viruses are naturally recognized by innate defense elements which draws the attention of the immune system. Besides their intrinsic immune stimulating properties, the adenoviruses used here are armed to express tumor necrosis factor alpha (TNFa) and interleukin-2 (IL-2). These cytokines result in immunological danger signaling and multiple appealing T-cell effects, including trafficking, activation and propagation. When these viruses were injected into B16.OVA melanoma tumors in animals concomitantly receiving programmed cell-death protein 1 (PD-1) blocking antibodies both tumor growth control (p < 0.0001) and overall survival (p < 0.01) were improved. In this set-up, the addition of adoptive cell therapy with OT-I lymphocytes did not increase efficacy further. When virus injections were initiated before antibody treatment in a prime-boost approach, 100% of tumors regressed completely and all mice survived. Viral expression of IL2 and TNFa altered the cytokine balance in the tumor microenvironment towards Th1 and increased the intratumoral proportion of CD8+ and conventional CD4+ T cells. These preclinical studies provide the rationale and schedule for a clinical trial where oncolytic adenovirus coding for TNFa and IL-2 (TILT-123) is used in melanoma patients receiving an anti-PD-1 antibody.
Keywords: Adenovirus; Adoptive T cell therapies; Adoptive cell therapy; Checkpoint blockade; Immunotherapy; Immunovirotherapy; Melanoma; Models of immunostimulation; Solid tumors; T cell therapy; Therapeutic antibodies; Virotherapy; anti-PD1.
Figures




Similar articles
-
Adenovirus Armed With TNFa and IL2 Added to aPD-1 Regimen Mediates Antitumor Efficacy in Tumors Refractory to aPD-1.Front Immunol. 2021 Jul 23;12:706517. doi: 10.3389/fimmu.2021.706517. eCollection 2021. Front Immunol. 2021. PMID: 34367166 Free PMC article.
-
Engineering Newcastle Disease Virus as an Oncolytic Vector for Intratumoral Delivery of Immune Checkpoint Inhibitors and Immunocytokines.J Virol. 2020 Jan 17;94(3):e01677-19. doi: 10.1128/JVI.01677-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694938 Free PMC article.
-
Tumor microenvironment remodeling by an engineered oncolytic adenovirus results in improved outcome from PD-L1 inhibition.Oncoimmunology. 2020 May 22;9(1):1761229. doi: 10.1080/2162402X.2020.1761229. Oncoimmunology. 2020. PMID: 32923123 Free PMC article.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Oncolytic adenoviruses: a game changer approach in the battle between cancer and the immune system.Expert Opin Biol Ther. 2019 May;19(5):443-455. doi: 10.1080/14712598.2019.1595582. Epub 2019 Mar 25. Expert Opin Biol Ther. 2019. PMID: 30905206 Review.
Cited by
-
Enhanced anti-melanoma efficacy through a combination of the armed oncolytic adenovirus ZD55-IL-24 and immune checkpoint blockade in B16-bearing immunocompetent mouse model.Cancer Immunol Immunother. 2021 Dec;70(12):3541-3555. doi: 10.1007/s00262-021-02946-z. Epub 2021 Apr 26. Cancer Immunol Immunother. 2021. PMID: 33903973 Free PMC article.
-
Inflammatory Regulation by TNF-α-Activated Adipose-Derived Stem Cells in the Human Bladder Cancer Microenvironment.Int J Mol Sci. 2021 Apr 13;22(8):3987. doi: 10.3390/ijms22083987. Int J Mol Sci. 2021. PMID: 33924332 Free PMC article.
-
Construction and application of adenoviral vectors.Mol Ther Nucleic Acids. 2023 Sep 9;34:102027. doi: 10.1016/j.omtn.2023.09.004. eCollection 2023 Dec 12. Mol Ther Nucleic Acids. 2023. PMID: 37808925 Free PMC article. Review.
-
Combination therapy with CAR T cells and oncolytic viruses: a new era in cancer immunotherapy.Cancer Gene Ther. 2022 Jun;29(6):647-660. doi: 10.1038/s41417-021-00359-9. Epub 2021 Jun 22. Cancer Gene Ther. 2022. PMID: 34158626 Review.
-
Combination of low-intensity pulsed ultrasound irradiating immune organs with immune checkpoint blockade augments systemic anti-tumor immunity on low tumor burden 4T-1 breast cancer.Cancer Immunol Immunother. 2025 Aug 6;74(9):281. doi: 10.1007/s00262-025-04137-6. Cancer Immunol Immunother. 2025. PMID: 40768050 Free PMC article.
References
-
- Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, Chastain M, Gorski KS, Anderson A, Chou J, et al.. Talimogene Laherparepvec in Combination With Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma. J Clin Oncol. 2016;34:2619–26. doi:10.1200/JCO.2016.67.1529. PMID:27298410 - DOI - PMC - PubMed
-
- Guarinos C, Juarez M, Egoavil C, Rodríguez-Soler M, Pérez-Carbonell L, Salas R, Cubiella J, Rodríguez-Moranta F, de-Castro L, Bujanda L, et al.. Prevalence and characteristics of MUTYH-associated polyposis in patients with multiple adenomatous and serrated polyps. Clin Cancer Res. 2014;20:1158–68. doi:10.1158/1078-0432.CCR-13-1490. PMID:24470512 - DOI - PubMed
-
- Quereux G, Pandolfino MC, Knol AC, Khammari A, Volteau C, Nguyen JM, Dreno B. Tissue prognostic markers for adoptive immunotherapy in melanoma. Eur J Dermatol. 2007;17:295–301. PMID:17540635 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
