CD96 targeted antibodies need not block CD96-CD155 interactions to promote NK cell anti-metastatic activity
- PMID: 29721390
- PMCID: PMC5927540
- DOI: 10.1080/2162402X.2018.1424677
CD96 targeted antibodies need not block CD96-CD155 interactions to promote NK cell anti-metastatic activity
Abstract
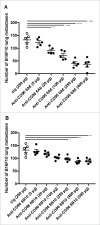
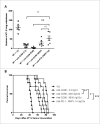
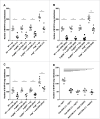
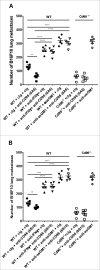
CD96 is a transmembrane glycoprotein Ig superfamily receptor, expressed on various T cell subsets and NK cells, that interacts with nectin and nectin-like proteins, including CD155/polio virus receptor (PVR). Here, we have compared three rat anti-mouse CD96 mAbs, including two that block CD96-CD155 (3.3 and 6A6) and one that does not block CD96-CD155 (8B10). Using flow cytometry, we demonstrated that both mAbs 3.3 and 6A6 bind to the first Ig domain of mouse CD96 and compete with CD155 binding, while mAb 8B10 binds to the second Ig domain and does not block CD155. While Fc isotype was irrelevant concerning the anti-metastatic activity of 3.3 mAb, in four different experimental metastases models and one spontaneous metastasis model, the relative order of anti-metastatic potency was 6A6 > 3.3 > 8B10. The metastatic burden control of all of the anti-CD96 clones was highly dependent on NK cells and IFN-γ. Consistent with its inability to block CD96-CD155 interactions, 8B10 retained anti-metastatic activity in CD155-deficient mice, whereas 3.3 and 6A6 lost potency in CD155-deficient mice. Furthermore, 8B10 retained most of its anti-metastatic activity in IL-12p35-deficient mice whereas the activity of 3.3 and 6A6 were partially lost. All three mAbs were inactive in CD226-deficient mice. Altogether, these data demonstrate anti-CD96 need not block CD96-CD155 interactions (ie. immune checkpoint blockade) to promote NK cell anti-metastatic activity.
Keywords: CD155; CD96; NK cells; immunotherapy; metastasis.
Figures



Similar articles
-
CD96 interaction with CD155 via its first Ig-like domain is modulated by alternative splicing or mutations in distal Ig-like domains.J Biol Chem. 2009 Jan 23;284(4):2235-44. doi: 10.1074/jbc.M807698200. Epub 2008 Dec 4. J Biol Chem. 2009. PMID: 19056733
-
Interaction between nectin-1 and the human natural killer cell receptor CD96.PLoS One. 2019 Feb 13;14(2):e0212443. doi: 10.1371/journal.pone.0212443. eCollection 2019. PLoS One. 2019. PMID: 30759143 Free PMC article.
-
Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy.Cancer Discov. 2016 Apr;6(4):446-59. doi: 10.1158/2159-8290.CD-15-0944. Epub 2016 Jan 19. Cancer Discov. 2016. PMID: 26787820
-
Coming of Age: CD96 Emerges as Modulator of Immune Responses.Front Immunol. 2018 May 17;9:1072. doi: 10.3389/fimmu.2018.01072. eCollection 2018. Front Immunol. 2018. PMID: 29868026 Free PMC article. Review.
-
Tumor intrinsic and extrinsic immune functions of CD155.Semin Cancer Biol. 2020 Oct;65:189-196. doi: 10.1016/j.semcancer.2019.11.013. Epub 2019 Dec 26. Semin Cancer Biol. 2020. PMID: 31883911 Review.
Cited by
-
Natural Killer Cell Engagers (NKCEs): a new frontier in cancer immunotherapy.Front Immunol. 2023 Aug 9;14:1207276. doi: 10.3389/fimmu.2023.1207276. eCollection 2023. Front Immunol. 2023. PMID: 37638058 Free PMC article. Review.
-
CD226 regulates natural killer cell antitumor responses via phosphorylation-mediated inactivation of transcription factor FOXO1.Proc Natl Acad Sci U S A. 2018 Dec 11;115(50):E11731-E11740. doi: 10.1073/pnas.1814052115. Epub 2018 Nov 30. Proc Natl Acad Sci U S A. 2018. PMID: 30504141 Free PMC article.
-
Immunological Targets for Immunotherapy: Inhibitory T Cell Receptors.Methods Mol Biol. 2020;2055:23-60. doi: 10.1007/978-1-4939-9773-2_2. Methods Mol Biol. 2020. PMID: 31502146 Free PMC article. Review.
-
Emergence of the CD226 Axis in Cancer Immunotherapy.Front Immunol. 2022 Jun 24;13:914406. doi: 10.3389/fimmu.2022.914406. eCollection 2022. Front Immunol. 2022. PMID: 35812451 Free PMC article. Review.
-
CD96, a new immune checkpoint, correlates with immune profile and clinical outcome of glioma.Sci Rep. 2020 Jul 1;10(1):10768. doi: 10.1038/s41598-020-66806-z. Sci Rep. 2020. PMID: 32612110 Free PMC article.
References
-
- Blake SJ, Stannard K, Liu J, Allen S, Yong MC, Mittal D, Aguilera AR, Miles JJ, Lutzky VP, de Andrade LF, et al.. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 2016;6:446–59. doi:10.1158/2159-8290.CD-15-0944. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
