Limited clinical utility of HLA-Cw6 genotyping for outcome prediction in psoriasis patients under ustekinumab therapy: a monocentric, retrospective analysis
- PMID: 29721444
- PMCID: PMC5919162
- DOI: 10.2147/PTT.S161437
Limited clinical utility of HLA-Cw6 genotyping for outcome prediction in psoriasis patients under ustekinumab therapy: a monocentric, retrospective analysis
Abstract
Purpose: Several studies have suggested that an HLA-Cw6+ allele can predict an improved outcome of treatment in psoriasis patients. The aim of the study was to assess whether the published association between HLA-Cw6 allele carriers and response to ustekinumab has the potential to impact treatment decisions.
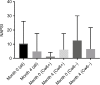
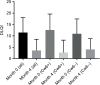
Patients and methods: Differences in Psoriasis Activity and Severity Index 50, 75, and 90; Nail Psoriasis Severity Index; and Dermatology Life Quality Index at 16 weeks were evaluated between HLA-Cw6 allele carriers vs. non-carriers. Thirty patients with moderate-to-severe psoriasis under treatment with ustekinumab were included in our study.
Results: There was no difference between the two groups with respect to Psoriasis Activity and Severity Index 50, 75, and 90 or in terms of change in Nail Psoriasis Severity Index or Dermatology Life Quality Index.
Conclusion: In our retrospectively analyzed cohort, we could not detect the previously reported better response in HLA-Cw6+ vs. HLA-Cw6- patients.
Keywords: Cw6; HLA-Cw6; genetic variations; human leukocyte antigen; ustekinumab.
Conflict of interest statement
Disclosure AAN is funded by the Promedica and Bruno - Bloch Foundation. He is on the advisory board of Janssen, AbbVie, Novartis, Celgene, MSD, Pfizer. FA is funded by Hochspezialisierte Medizin 2 from the canton of Zurich; he is also funded by Forschungskredit of the University of Zurich. FA received honoraria from AbbVie, Novartis, Celgene and Eli Lilly. The authors report no other conflicts of interest in this work.
Figures

References
-
- O’Rielly DD, Rahman P. Genetics of psoriatic arthritis. Best Pract Res Clin Rheumatol. 2014;28(5):673–685. - PubMed
-
- Duffin KC, Woodcock J, Krueger GG. Genetic variations associated with psoriasis and psoriatic arthritis found by genome-wide association. Dermatol Ther. 2010;23(2):101–113. - PubMed
-
- Mabuchi T, Ota T, Manabe Y, et al. HLA-C*12:02 is a susceptibility factor in late-onset type of psoriasis in Japanese. J Dermatol. 2014;41(8):697–704. - PubMed
-
- Ruiz-Romeu E, Ferran M, Sagrista M, et al. Streptococcus pyogenes-induced cutaneous lymphocyte antigen-positive T cell-dependent epidermal cell activation triggers TH17 responses in patients with guttate psoriasis. J Allergy Clin Immunol. 2016;138(2):491–499.e496. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

