Hippocampal PPARα is a novel therapeutic target for depression and mediates the antidepressant actions of fluoxetine in mice
- PMID: 29722018
- PMCID: PMC6016645
- DOI: 10.1111/bph.14346
Hippocampal PPARα is a novel therapeutic target for depression and mediates the antidepressant actions of fluoxetine in mice
Abstract
Background and purpose: Developing novel pharmacological targets beyond the monoaminergic system is now a popular strategy for treating depression. PPARα is a nuclear receptor protein that functions as a transcription factor,-regulating gene expression. We have previously reported that both WY14643 and fenofibrate, two pharmacological agonists of PPARα, have antidepressant-like effects in mice, implying that PPARα is a potential antidepressant target.
Experimental approach: We first used various biotechnological methods to evaluate the effects of chronic stress and fluoxetine on hippocampal PPARα. The viral-mediated genetic approach was then employed to explore whether hippocampal PPARα was an antidepressant target. PPARα inhibitors, PPARα-knockout (KO) mice and PPARα-knockdown (KD) mice were further used to determine the role of PPARα in the antidepressant effects of fluoxetine.
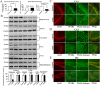
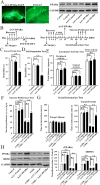
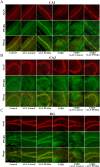
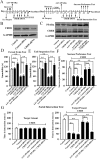
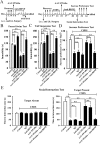
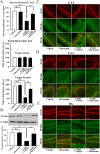
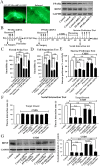
Key results: Chronic stress significantly decreased mRNA and protein levels of PPARα in the hippocampus, but not other regions, and also fully reduced the recruitment of hippocampal PPARα to the cAMP response element-binding (CREB) promoter. Genetic overexpression of hippocampal PPARα induced significant antidepressant-like actions in mice by promoting CREB-mediated biosynthesis of brain-derived neurotrophic factor. Moreover, fluoxetine notably restored the stress-induced negative effects on hippocampal PPARα. Using PPARα antagonists fully blocked the antidepressant effects of fluoxetine in mice, and similarly, both PPARα-KO and PPARα-KD abolished the effects of fluoxetine. Besides, PPARα-KO and PPARα-KD aggravated depression in mice.
Conclusions and implications: Hippocampal PPARα is a potential novel antidepressant target that mediates the antidepressant actions of fluoxetine in mice.
© 2018 The British Pharmacological Society.
Figures


Similar articles
-
Hippocampal PPARα is involved in the antidepressant-like effects of venlafaxine in mice.Brain Res Bull. 2019 Nov;153:171-180. doi: 10.1016/j.brainresbull.2019.08.016. Epub 2019 Aug 21. Brain Res Bull. 2019. PMID: 31445056
-
Hippocampal Salt-Inducible Kinase 2 Plays a Role in Depression via the CREB-Regulated Transcription Coactivator 1-cAMP Response Element Binding-Brain-Derived Neurotrophic Factor Pathway.Biol Psychiatry. 2019 Apr 15;85(8):650-666. doi: 10.1016/j.biopsych.2018.10.004. Epub 2018 Oct 18. Biol Psychiatry. 2019. PMID: 30503507
-
Promoting the hippocampal PPARα expression participates in the antidepressant mechanism of reboxetine, a selective norepinephrine reuptake inhibitor.Behav Brain Res. 2022 Jan 7;416:113535. doi: 10.1016/j.bbr.2021.113535. Epub 2021 Aug 18. Behav Brain Res. 2022. PMID: 34416301
-
[Anti-apoptotic protein enhances resilience to psychoemotional effects of stress].Usp Fiziol Nauk. 2013 Apr-Jun;44(2):3-13. Usp Fiziol Nauk. 2013. PMID: 23789349 Review. Russian.
-
Bicaudal-C protein, a potential antidepressant target.Neuroreport. 2021 Nov 2;32(16):1293-1298. doi: 10.1097/WNR.0000000000001729. Neuroreport. 2021. PMID: 34554934 Review.
Cited by
-
Differential and paradoxical roles of new-generation antidepressants in primary astrocytic inflammation.J Neuroinflammation. 2021 Feb 18;18(1):47. doi: 10.1186/s12974-021-02097-z. J Neuroinflammation. 2021. PMID: 33602262 Free PMC article.
-
Central Adiponectin Signaling - A Metabolic Regulator in Support of Brain Plasticity.Brain Plast. 2022 Oct 21;8(1):79-96. doi: 10.3233/BPL-220138. eCollection 2022. Brain Plast. 2022. PMID: 36448043 Free PMC article. Review.
-
Activation of mTORC1 Signaling Cascade in Hippocampus and Medial Prefrontal Cortex Is Required for Antidepressant Actions of Vortioxetine in Mice.Int J Neuropsychopharmacol. 2023 Oct 19;26(10):655-668. doi: 10.1093/ijnp/pyad017. Int J Neuropsychopharmacol. 2023. PMID: 37025079 Free PMC article.
-
Administration of chiglitazar reverses chronic stress-induced depressive-like symptoms in mice via activation of hippocampal PPARα and BDNF.Front Pharmacol. 2025 Apr 28;16:1587399. doi: 10.3389/fphar.2025.1587399. eCollection 2025. Front Pharmacol. 2025. PMID: 40356991 Free PMC article.
-
Indole-3-Carbinol Selectively Prevents Chronic Stress-Induced Depression-but not Anxiety-Like Behaviors via Suppressing Pro-Inflammatory Cytokine Production and Oxido-Nitrosative Stress in the Brain.Front Pharmacol. 2022 Feb 15;13:829966. doi: 10.3389/fphar.2022.829966. eCollection 2022. Front Pharmacol. 2022. PMID: 35242039 Free PMC article.
References
-
- Agarwal S, Yadav A, Chaturvedi RK (2017). Peroxisome proliferator‐activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem Biophys Res Commun 483: 1166–1177. - PubMed
-
- Barbiero JK, Santiago R, Tonin FS, Boschen S, da Silva LM, Werner MF et al (2014). PPAR‐α agonist fenofibrate protects against the damaging effects of MPTP in a rat model of Parkinson's disease. Prog Neuro‐Psychopharmacol Biol Psychiatry 53: 35–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

