A regulatory loop connecting WNT signaling and telomere capping: possible therapeutic implications for dyskeratosis congenita
- PMID: 29722029
- PMCID: PMC7899295
- DOI: 10.1111/nyas.13692
A regulatory loop connecting WNT signaling and telomere capping: possible therapeutic implications for dyskeratosis congenita
Abstract
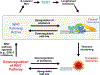
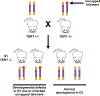
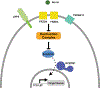
The consequences of telomere dysfunction are most apparent in rare inherited syndromes caused by genetic deficiencies in factors that normally maintain telomeres. The principal disease is known as dyskeratosis congenita (DC), but other syndromes with similar underlying genetic defects share some clinical aspects with this disease. Currently, there are no curative therapies for these diseases of telomere dysfunction. Here, we review recent findings demonstrating that dysfunctional (i.e., uncapped) telomeres can downregulate the WNT pathway, and that restoration of WNT signaling helps to recap telomeres by increasing expression of shelterins, proteins that naturally bind and protect telomeres. We discuss how these findings are different from previous observations connecting WNT and telomere biology, and discuss potential links between WNT and clinical manifestations of the DC spectrum of diseases. Finally, we argue for exploring the use of WNT agonists, specifically lithium, as a possible therapeutic approach for patients with DC.
Keywords: WNT; dyskeratosis congenita; lithium; pulmonary fibrosis; telomerase; telomeres.
© 2018 New York Academy of Sciences.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

Similar articles
-
Impaired Telomere Maintenance and Decreased Canonical WNT Signaling but Normal Ribosome Biogenesis in Induced Pluripotent Stem Cells from X-Linked Dyskeratosis Congenita Patients.PLoS One. 2015 May 18;10(5):e0127414. doi: 10.1371/journal.pone.0127414. eCollection 2015. PLoS One. 2015. PMID: 25992652 Free PMC article.
-
GSK3 inhibition rescues growth and telomere dysfunction in dyskeratosis congenita iPSC-derived type II alveolar epithelial cells.Elife. 2022 May 13;11:e64430. doi: 10.7554/eLife.64430. Elife. 2022. PMID: 35559731 Free PMC article.
-
Recent progress in dyskeratosis congenita.Int J Hematol. 2010 Oct;92(3):419-24. doi: 10.1007/s12185-010-0695-5. Epub 2010 Oct 1. Int J Hematol. 2010. PMID: 20882440 Review.
-
Dyskeratosis congenita -- a disease of dysfunctional telomere maintenance.Curr Mol Med. 2005 Mar;5(2):159-70. doi: 10.2174/1566524053586581. Curr Mol Med. 2005. PMID: 15974869 Review.
-
Enhancing a Wnt-Telomere Feedback Loop Restores Intestinal Stem Cell Function in a Human Organotypic Model of Dyskeratosis Congenita.Cell Stem Cell. 2016 Sep 1;19(3):397-405. doi: 10.1016/j.stem.2016.05.024. Epub 2016 Aug 18. Cell Stem Cell. 2016. PMID: 27545506 Free PMC article.
Cited by
-
Non-canonical Roles of Telomerase: Unraveling the Imbroglio.Front Cell Dev Biol. 2019 Dec 10;7:332. doi: 10.3389/fcell.2019.00332. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31911897 Free PMC article. Review.
-
Exploring the Interplay of Telomerase Reverse Transcriptase and β-Catenin in Hepatocellular Carcinoma.Cancers (Basel). 2021 Aug 20;13(16):4202. doi: 10.3390/cancers13164202. Cancers (Basel). 2021. PMID: 34439356 Free PMC article. Review.
-
Telomere length and hTERT genetic variants as potential prognostic markers in multiple myeloma.Sci Rep. 2023 Sep 22;13(1):15792. doi: 10.1038/s41598-023-43141-7. Sci Rep. 2023. PMID: 37737335 Free PMC article.
-
Special pre- and posttransplant considerations in inherited bone marrow failure and hematopoietic malignancy predisposition syndromes.Hematology Am Soc Hematol Educ Program. 2020 Dec 4;2020(1):107-114. doi: 10.1182/hematology.2020000095. Hematology Am Soc Hematol Educ Program. 2020. PMID: 33275667 Free PMC article. Review.
-
Understanding inborn errors of immunity: A lens into the pathophysiology of monogenic inflammatory bowel disease.Front Immunol. 2022 Sep 29;13:1026511. doi: 10.3389/fimmu.2022.1026511. eCollection 2022. Front Immunol. 2022. PMID: 36248828 Free PMC article. Review.
References
-
- Herbig U, Jobling WA, Chen BPC, et al. 2004. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14: 501–513. - PubMed
-
- Harley CB, Futcher AB & Greider CW. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

