Administration of Cripto in GRP78 overexpressed human MSCs enhances stem cell viability and angiogenesis during human MSC transplantation therapy
- PMID: 29722092
- PMCID: PMC6528945
- DOI: 10.1111/cpr.12463
Administration of Cripto in GRP78 overexpressed human MSCs enhances stem cell viability and angiogenesis during human MSC transplantation therapy
Abstract
Objectives: The purpose of this study was to explore the effectiveness of concurrent GRP78 overexpression combined with Cripto on hMSC proliferation and migration both in vitro and in vivo. Specifically, we explored whether the treatment enhances effectiveness of hMSC transplantation in ischaemic tissue.
Materials and methods: Human MSCs obtained from human adipose tissue were cultured in α-minimum essential medium (Hyclone, Logan, UT, USA) supplemented with 10% (v/v) foetal bovine serum (Hyclone), 100 U mL-1 penicillin and 100 μg mL-1 streptomycin. Murine hindlimb ischaemic model was generated with 8-week-old male nude BALB/c mice (Biogenomics, Seoul, Korea) maintained under a 12-h light/dark cycle following the established protocol with minor modification. Cellular injection was performed no later than 3 hour after surgery. Lipofectamine transfection, single-cell cultivation assay, transwell assay, scratch wound-healing migration assay, immunohistochemistry and western blotting assays were performed.
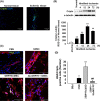
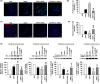
Results: Overexpression of GRP78 along with Cripto enhanced hMSC proliferation, migration and invasion. It increased interaction of surface GRP78 receptor with Cripto via JAK2/STAT3 pathway. We confirmed our proposed mechanism by showing that treatment with GRP78 antibody blocks the enhancement in vitro. In vivo, we observed that Cripto induced by the hypoxic environment in hindlimb ischaemic model interacts with the overexpressed GRP78 and increases hMSC proliferation, migration and invasion potentials as well as angiogenesis around transplanted ischaemic site via cytokine secretions.
Conclusions: These results demonstrate supporting evidences that GRP78-Cripto combination technique offers novel strategy to enhance MSC proliferation, migration and invasion potentials as well as angiogenesis around ischaemic site, ultimately facilitating MSC-based transplantation therapy in ischaemic conditions.
Keywords: Cripto; GRP78; angiogenesis; cell proliferation; invasion; mesenchymal stem cells.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Nakagami H, Maeda K, Morishita R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue‐derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542‐2547. - PubMed
-
- Vaananen HK. Mesenchymal stem cells. Ann Med. 2005;37:469‐479. - PubMed
-
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25:2896‐2902. - PubMed
-
- Potapova IA, Gaudette GR, Brink PR, et al. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761‐1768. - PubMed
-
- Andrades JA, Han B, Becerra J, Sorgente N, Hall FL, Nimni ME. A recombinant human TGF‐beta1 fusion protein with collagen‐binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cells. Exp Cell Res. 1999;250:485‐498. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

