Five year neurodevelopment outcomes of perinatally HIV-infected children on early limited or deferred continuous antiretroviral therapy
- PMID: 29722482
- PMCID: PMC5932637
- DOI: 10.1002/jia2.25106
Five year neurodevelopment outcomes of perinatally HIV-infected children on early limited or deferred continuous antiretroviral therapy
Abstract
Introduction: Early antiretroviral therapy (ART) has improved neurodevelopmental outcomes of HIV-infected (HIV-positive) children; however, little is known about the longer term outcomes in infants commencing early ART or whether temporary ART interruption might have long-term consequences. In the children with HIV early antiretroviral treatment (CHER) trial, HIV-infected infants ≤12 weeks of age with CD4 ≥25% were randomized to deferred ART (ART-Def); immediate time-limited ART for 40 weeks (ART-40W) or 96 weeks (ART-96W). ART was restarted in the time-limited arms for immunologic/clinical progression. Our objective was to compare the neurodevelopmental profiles in all three arms of Cape Town CHER participants.
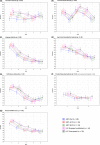
Methods: A prospective, longitudinal observational study was used. The Griffiths mental development scales (GMDS), which includes six subscales and a global score, were performed at 11, 20, 30, 42 and 60 months, and the Beery-Buktenica developmental tests for visual motor integration at 60 months. HIV-exposed uninfected (HEU) and HIV-unexposed (HU) children were enrolled for comparison. Mixed model repeated measures were used to compare groups over time, using quotients derived from standardized British norms.
Results: In this study, 28 ART-Def, 35 ART-40W, 33 ART-96W CHER children, and 34 HEU and 39 HU controls were enrolled. GMDS scores over five years were similar between the five groups in all subscales except locomotor and general Griffiths (interaction p < 0.001 and p = 0.02 respectively), driven by early lower scores in the ART-Def arm. At 60 months, scores for all groups were similar in each GMDS scale. However, Beery visual perception scores were significantly lower in HIV-infected children (mean standard scores: 75.8 ART-Def, 79.8 ART-40W, 75.9 ART-96W) versus 84.4 in HEU and 90.5 in HU (p < 0.01)).
Conclusions: Early locomotor delay in the ART-Def arm resolved by five years. Neurodevelopmental outcomes at five years in HIV-infected children on early time-limited ART were similar to uninfected controls, apart from visual perception where HIV-infected children scored lower. Poorer visual perception performance warrants further investigation.
Keywords: ARV; CHER trial; Children; Early time-limited antiretroviral therapy; Griffiths mental development scales; HIV care continuum; Neurodevelopment; Treatment interruption; Visual perception.
© 2018 The Authors. Journal of the International AIDS Society published by John Wiley & sons Ltd on behalf of the International AIDS Society.
Figures
References
-
- Le Doare K, Bland R, Newell ML. Neurodevelopment in children born to HIV‐infected mothers by infection and treatment status. Pediatrics. 2012;130(5):e1326–44. - PubMed
-
- Faye A, Le Chenadec J, Dollfus C, Thuret I, Douard D, Firtion G, et al. Early versus deferred antiretroviral multidrug therapy in infants infected with HIV type 1. Clin Infect Dis. 2004;39(11):1692–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

