Long-term culture and cloning of primary human bronchial basal cells that maintain multipotent differentiation capacity and CFTR channel function
- PMID: 29722564
- PMCID: PMC6139663
- DOI: 10.1152/ajplung.00355.2017
Long-term culture and cloning of primary human bronchial basal cells that maintain multipotent differentiation capacity and CFTR channel function
Abstract
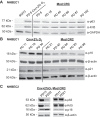
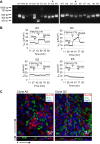
While primary cystic fibrosis (CF) and non-CF human bronchial epithelial basal cells (HBECs) accurately represent in vivo phenotypes, one barrier to their wider use has been a limited ability to clone and expand cells in sufficient numbers to produce rare genotypes using genome-editing tools. Recently, conditional reprogramming of cells (CRC) with a Rho-associated protein kinase (ROCK) inhibitor and culture on an irradiated fibroblast feeder layer resulted in extension of the life span of HBECs, but differentiation capacity and CF transmembrane conductance regulator (CFTR) function decreased as a function of passage. This report details modifications to the standard HBEC CRC protocol (Mod CRC), including the use of bronchial epithelial cell growth medium, instead of F medium, and 2% O2, instead of 21% O2, that extend HBEC life span while preserving multipotent differentiation capacity and CFTR function. Critically, Mod CRC conditions support clonal growth of primary HBECs from a single cell, and the resulting clonal HBEC population maintains multipotent differentiation capacity, including CFTR function, permitting gene editing of these cells. As a proof-of-concept, CRISPR/Cas9 genome editing and cloning were used to introduce insertions/deletions in CFTR exon 11. Mod CRC conditions overcome many barriers to the expanded use of HBECs for basic research and drug screens. Importantly, Mod CRC conditions support the creation of isogenic cell lines in which CFTR is mutant or wild-type in the same genetic background with no history of CF to enable determination of the primary defects of mutant CFTR.
Keywords: CRISPR; ROCK inhibitor; conditional reprogramming; cystic fibrosis; human bronchial epithelial cells.
Figures







References
-
- Boat TF, Welsh MJ, Beaudet AL. Cystic Fibrosis. New York: McGraw-Hill, 1989.
-
- Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML, Hawkins F, Liao W, Mora D, Choi S, Wang J, Sun HC, Paschon DE, Guschin DY, Gregory PD, Kotton DN, Holmes MC, Sorscher EJ, Davis BR. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Reports 4: 569–577, 2015. doi: 10.1016/j.stemcr.2015.02.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

