Phase 2 Study of Bortezomib Combined With Temozolomide and Regional Radiation Therapy for Upfront Treatment of Patients With Newly Diagnosed Glioblastoma Multiforme: Safety and Efficacy Assessment
- PMID: 29722661
- PMCID: PMC6277207
- DOI: 10.1016/j.ijrobp.2018.01.001
Phase 2 Study of Bortezomib Combined With Temozolomide and Regional Radiation Therapy for Upfront Treatment of Patients With Newly Diagnosed Glioblastoma Multiforme: Safety and Efficacy Assessment
Erratum in
-
Erratum to: Kong X-T, Nguyen NT, Choi YJ, et al. Phase 2 Study of Bortezomib Combined With Temozolomide and Regional Radiation Therapy for Upfront Treatment of Patients With Newly Diagnosed Glioblastoma Multiforme: Safety and Efficacy Assessment. Int J Radiat Oncol Biol Phys 2018;100:1195-1203.Int J Radiat Oncol Biol Phys. 2019 Apr 1;103(5):1289. doi: 10.1016/j.ijrobp.2019.01.071. Epub 2019 Mar 13. Int J Radiat Oncol Biol Phys. 2019. PMID: 30900570 No abstract available.
Abstract
Purpose: To assess the safety and efficacy of upfront treatment using bortezomib combined with standard radiation therapy (RT) and temozolomide (TMZ), followed by adjuvant bortezomib and TMZ for ≤24 cycles, in patients with newly diagnosed glioblastoma multiforme (GBM).
Methods and materials: Twenty-four patients with newly diagnosed GBM were enrolled. The patients received standard external beam regional RT with concurrent TMZ beginning 3 to 6 weeks after surgery, followed by adjuvant TMZ and bortezomib for ≤24 cycles or until tumor progression. During RT, bortezomib was given at 1.3 mg/m2 on days 1, 4, 8, 11, 29, 32, 36, and 39. After RT, bortezomib was given at 1.3 mg/m2 on days 1, 4, 8, and 11 every 4 weeks.
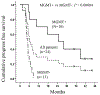
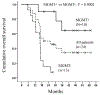
Results: No unexpected adverse events occurred from the addition of bortezomib. The efficacy analysis showed a median progression-free survival (PFS) of 6.2 months (95% confidence interval [CI] 3.7-8.8), with promising PFS rates at ≥18 months compared with historical norms (25.0% at 18 and 24 months; 16.7% at 30 months). In terms of overall survival (OS), the median OS was 19.1 months (95% CI 6.7-31.4), with improved OS rates at ≥12 months (87.5% at 12, 50.0% at 24, 34.1% at 36-60 months) compared with the historical norms. The median PFS was 24.7 months (95% CI 8.5-41.0) in 10 MGMT methylated and 5.1 months (95% CI 3.9-6.2) in 13 unmethylated patients. The estimated median OS was 61 months (95% CI upper bound not reached) in the methylated and 16.4 months (95% CI 11.8-21.0) in the unmethylated patients.
Conclusions: The addition of bortezomib to current standard radiochemotherapy in newly diagnosed GBM patients was tolerable. The PFS and OS rates appeared promising, with more benefit to MGMT methylated patients. Further clinical investigation is warranted in a larger cohort of patients.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest:
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Xiao-Tang Kong MD PhD
Nhung T. Nguyen
Yoon J. Choi MD
Guicheng Zhang, MD PhD
HuyTram N. Nguyen
Emese Filka MD
Stacey Green MSN
P. Leia Nghiemphu MD
William H. Yong MD
Linda M. Liau MD PhD
Tania Kaprealian MD
Whitney B. Pope MD PhD
Andrew Lassman, MD
Tim Cloughesy MD
The authors whose names are listed immediately below report the following details of affiliation or involvement in an organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this manuscript. Please specify the nature of the conflict on a separate sheet of paper if the space below is inadequate.
Albert Lai, MD, PhD – Grant from Millennium/Takeda (This is an investigator initiated industry sponsored study)
**Author disclosure forms from all authors were uploaded.
Figures
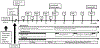
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996 - PubMed
-
- Chinot OL, Wick W, and Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–722 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

