The nitric oxide-guanylate cyclase pathway and glaucoma
- PMID: 29723581
- PMCID: PMC6424573
- DOI: 10.1016/j.niox.2018.04.010
The nitric oxide-guanylate cyclase pathway and glaucoma
Abstract
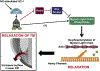
Glaucoma is a prevalent optic neuropathy characterized by the progressive dysfunction and loss of retinal ganglion cells (RGCs) and their optic nerve axons, which leads to irreversible visual field loss. Multiple risk factors for the disease have been identified, but elevated intraocular pressure (IOP) remains the primary risk factor amenable to treatment. Reducing IOP however does not always prevent glaucomatous neurodegeneration, and many patients progress with the disease despite having IOP in the normal range. There is increasing evidence that nitric oxide (NO) is a direct regulator of IOP and that dysfunction of the NO-Guanylate Cyclase (GC) pathway is associated with glaucoma incidence. NO has shown promise as a novel therapeutic with targeted effects that: 1) lower IOP; 2) increase ocular blood flow; and 3) confer neuroprotection. The various effects of NO in the eye appear to be mediated through the activation of the GC- guanosine 3:5'-cyclic monophosphate (cGMP) pathway and its effect on downstream targets, such as protein kinases and Ca2+ channels. Although NO-donor compounds are promising as therapeutics for IOP regulation, they may not be ideal to harness the neuroprotective potential of NO signaling. Here we review evidence that supports direct targeting of GC as a novel pleiotrophic treatment for the disease, without the need for direct NO application. The identification and targeting of other factors that contribute to glaucoma would be beneficial to patients, particularly those that do not respond well to IOP-dependent interventions.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures



References
-
- Tham YC, Li X, Wong TY, Quigley HA, Aung T and Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. - PubMed
-
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M and Early Manifest Glaucoma Trial G. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

