Assembly of Tissue-Engineered Blood Vessels with Spatially Controlled Heterogeneities
- PMID: 29724157
- PMCID: PMC6198764
- DOI: 10.1089/ten.TEA.2017.0492
Assembly of Tissue-Engineered Blood Vessels with Spatially Controlled Heterogeneities
Abstract
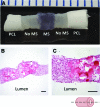
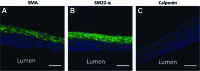
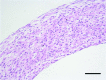
Tissue-engineered human blood vessels may enable in vitro disease modeling and drug screening to accelerate advances in vascular medicine. Existing methods for tissue-engineered blood vessel (TEBV) fabrication create homogenous tubes not conducive to modeling the focal pathologies characteristic of certain vascular diseases. We developed a system for generating self-assembled human smooth muscle cell (SMC) ring units, which were fused together into TEBVs. The goal of this study was to assess the feasibility of modular assembly and fusion of ring building units to fabricate spatially controlled, heterogeneous tissue tubes. We first aimed to enhance fusion and reduce total culture time, and determined that reducing ring preculture duration improved tube fusion. Next, we incorporated electrospun polymer ring units onto tube ends as reinforced extensions, which allowed us to cannulate tubes after only 7 days of fusion, and culture tubes with luminal flow in a custom bioreactor. To create focal heterogeneities, we incorporated gelatin microspheres into select ring units during self-assembly, and fused these rings between ring units without microspheres. Cells within rings maintained their spatial position along tissue tubes after fusion. Because tubes fabricated from primary SMCs did not express contractile proteins, we also fabricated tubes from human mesenchymal stem cells, which expressed smooth muscle alpha actin and SM22-α. This work describes a platform approach for creating modular TEBVs with spatially defined structural heterogeneities, which may ultimately be applied to mimic focal diseases such as intimal hyperplasia or aneurysm.
Keywords: bioreactor; electrospun cannulation cuff; gelatin microspheres; modular tissue engineering; tissue-engineered blood vessel; vascular disease model.
Conflict of interest statement
The authors have no competing financial interests.
Figures

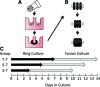

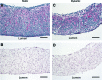
 ), tube length (L), and thickness (T) was measured for each sample on each day of culture (A). Fusion angles (B), tube length (C), and thickness (D) as a function of time for tubes fabricated from rings cultured for 3 (3–7), 5 (5–7), or 7 (7–7) days before 7 days of fusion culture. N = 3 tubes per group. Data points are mean ± SD. #p < 0.05 for 5–7 versus 3–7 and 7–7, ** p < 0.05 for 5–7 versus 7–7, *p < 0.05. Scale = 0.5 mm.
), tube length (L), and thickness (T) was measured for each sample on each day of culture (A). Fusion angles (B), tube length (C), and thickness (D) as a function of time for tubes fabricated from rings cultured for 3 (3–7), 5 (5–7), or 7 (7–7) days before 7 days of fusion culture. N = 3 tubes per group. Data points are mean ± SD. #p < 0.05 for 5–7 versus 3–7 and 7–7, ** p < 0.05 for 5–7 versus 7–7, *p < 0.05. Scale = 0.5 mm.
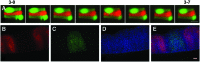
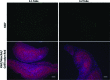

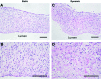
References
-
- Stegemann J.P., and Nerem R.M. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp Cell Res 283, 146, 2003 - PubMed
-
- Alexander J.H., Hafley G., Harrington R.A., et al. . Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. JAMA 294, 2446, 2005 - PubMed
-
- Kim F.Y., Marhefka G., Ruggiero N.J., Adams S., and Whellan D.J. Saphenous vein graft disease: review of pathophysiology, prevention, and treatment. Cardiol Rev 21, 101, 2013 - PubMed
-
- Wystrychowski W., McAllister T.N., Zagalski K., Dusserre N., Cierpka L., and L'Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg 60, 1353, 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

