The effect of spironolactone on calcineurin inhibitor induced nephrotoxicity: a multicenter randomized, double-blind, clinical trial (the SPIREN trial)
- PMID: 29724188
- PMCID: PMC5934785
- DOI: 10.1186/s12882-018-0885-6
The effect of spironolactone on calcineurin inhibitor induced nephrotoxicity: a multicenter randomized, double-blind, clinical trial (the SPIREN trial)
Abstract
Background: Calcineurin inhibitor induced nephrotoxicity contributes to late allograft failure in kidney transplant patients. Evidence points towards aldosterone to play a role in the development of fibrosis in multiple organs. Animal studies have indicated a beneficial effect of mineralocorticoid receptor antagonists preventing calcineurin inhibitor induced nephrotoxicity. Only few studies have explored this effect in humans. The objective of this study is to evaluate the effect of spironolactone on glomerular filtration rate and fibrosis in kidney transplant patients.
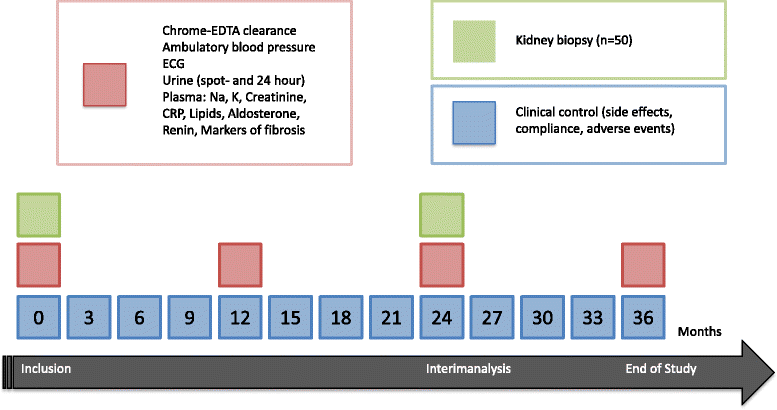
Method: Prospective, double-blind, randomized, clinical trial including 170 prevalent kidney transplant patients. Patients are randomized to spironolactone 25-50 mg/day or placebo for three years. Primary outcome is glomerular filtration rate evaluated by chrome-EDTA clearance. Secondary outcomes are 24-h protein excretion, amount of interstitial fibrosis in renal allograft biopsies, and cardiovascular events. As an exploratory outcome, we aim to identify markers of fibrosis in blood and urine.
Discussion: Long term allograft survival remains a key issue in renal transplantation, partly due to calcineurin inhibitor induced nephrotoxicity. Evidence from animal- and small human studies indicate a beneficial effect of mineralocorticoid receptor antagonism on renal function and fibrosis. This study aims to test this hypothesis in a sufficiently powered randomized clinical trial. Results might influence the future management of long term allograft survival in renal transplantation.
Trial registration: ClinicalTrials.gov identifier (05/17/2012): NCT01602861 . EudraCT number (05/31/2011): 2011-002243-98.
Keywords: Aldosterone; Cyclosporine A; Fibrosis; Glomerular filtration rate; IFTA; Kidney transplantation; Mineralocorticoid; Tacrolimus.
Conflict of interest statement
Ethics approval and consent to participate
The SPIREN trial has been approved by the Research Ethics Committee of Southern Denmark on the 24th of August 2011 (project ID: s-20110095, protocol version 2 (07/28/2011)). Oral and written informed consent to participation is obtained from all study participants by study personnel prior to any study related procedure. All participants receive both written and oral information about the study before giving consent. The trial is conducted according to the standards of Good Clinical Practice (GCP) and undergoes continuous monitoring by local GCP representatives.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials