Systemic inhibition of the membrane attack complex impedes neuroinflammation in chronic relapsing experimental autoimmune encephalomyelitis
- PMID: 29724241
- PMCID: PMC5932802
- DOI: 10.1186/s40478-018-0536-y
Systemic inhibition of the membrane attack complex impedes neuroinflammation in chronic relapsing experimental autoimmune encephalomyelitis
Abstract
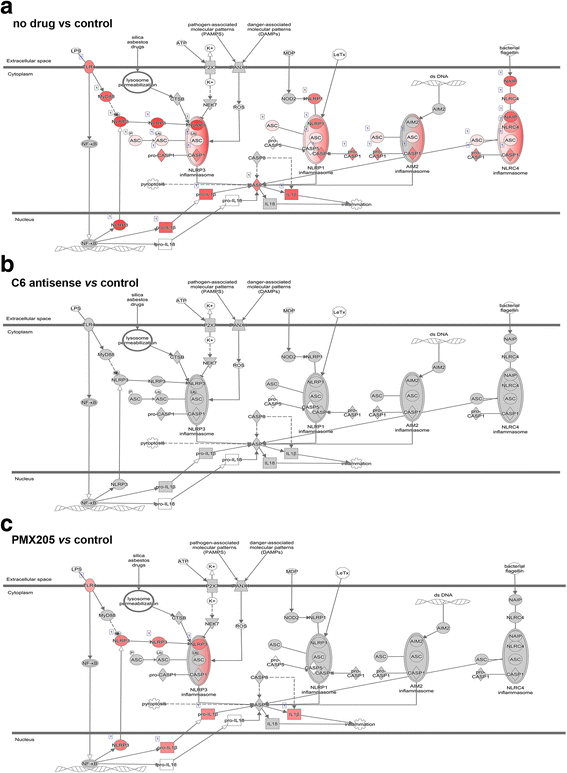
The complement system is a key driver of neuroinflammation. Activation of complement by all pathways, results in the formation of the anaphylatoxin C5a and the membrane attack complex (MAC). Both initiate pro-inflammatory responses which can contribute to neurological disease. In this study, we delineate the specific roles of C5a receptor signaling and MAC formation during the progression of experimental autoimmune encephalomyelitis (EAE)-mediated neuroinflammation. MAC inhibition was achieved by subcutaneous administration of an antisense oligonucleotide specifically targeting murine C6 mRNA (5 mg/kg). The C5a receptor 1 (C5aR1) was inhibited with the C5a receptor antagonist PMX205 (1.5 mg/kg). Both treatments were administered systemically and started after disease onset, at the symptomatic phase when lymphocytes are activated. We found that antisense-mediated knockdown of C6 expression outside the central nervous system prevented relapse of disease by impeding the activation of parenchymal neuroinflammatory responses, including the Nod-like receptor protein 3 (NLRP3) inflammasome. Furthermore, C6 antisense-mediated MAC inhibition protected from relapse-induced axonal and synaptic damage. In contrast, inhibition of C5aR1-mediated inflammation diminished expression of major pro-inflammatory mediators, but unlike C6 inhibition, it did not stop progression of neurological disability completely. Our study suggests that MAC is a key driver of neuroinflammation in this model, thereby MAC inhibition might be a relevant treatment for chronic neuroinflammatory diseases.
Keywords: Complement; Inflammasome; Neuroinflammation.
Conflict of interest statement
Ethics approval
All experiments were approved by the Academic Medical Center Animal Ethics Committee and complied with the Dutch national policy on humane care and the use of laboratory animals.
Competing interests
FB, KF and VR are the inventors of patents that describe the use of inhibitors of the terminal complement pathway for therapeutic purposes; they are co-founders of Regenesance BV. FB. is founder of Complement Pharma BV.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Arbore G, West EE, Spolski R, Robertson AAB, Klos A, Rheinheimer C, Dutow P, Woodruff TM, Yu ZX, O'Neill LA, Coll RC, Sher A, Leonard WJ, Kohl J, Monk P, Cooper MA, Arno M, Afzali B, Lachmann HJ, Cope AP, Mayer-Barber KD, Kemper C (2016) T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science 352:aad1210. doi:10.1126/science.aad1210 - PMC - PubMed
-
- Bahia El Idrissi N, Das PK, Fluiter K, Rosa PS, Vreijling J, Troost D, Morgan BP, Baas F, Ramaglia V. M. Leprae components induce nerve damage by complement activation: identification of lipoarabinomannan as the dominant complement activator. Acta Neuropathol. 2015;129:653–667. doi: 10.1007/s00401-015-1404-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

