Lighting a Fire in the Tumor Microenvironment Using Oncolytic Immunotherapy
- PMID: 29724655
- PMCID: PMC6013846
- DOI: 10.1016/j.ebiom.2018.04.020
Lighting a Fire in the Tumor Microenvironment Using Oncolytic Immunotherapy
Abstract
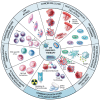
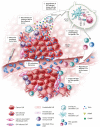
Oncolytic virus (OV) therapy is potentially a game-changing cancer treatment that has garnered significant interest due to its versatility and multi-modal approaches towards tumor eradication. In the field of cancer immunotherapy, the immunological phenotype of the tumor microenvironment (TME) is an important determinant of disease prognosis and therapeutic success. There is accumulating data that OVs are capable of dramatically altering the TME immune landscape, leading to improved antitumor activity alone or in combination with assorted immune modulators. Herein, we review how OVs disrupt the immunosuppressive TME and can be used strategically to create a "pro-immune" microenvironment that enables and promotes potent, long-lasting host antitumor immune responses.
Keywords: Cancer immunotherapies; Combination therapies; Oncolytic viruses; Tumor microenvironment.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. - PubMed
-
- Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. - PubMed
-
- Joyce J.A., Fearon D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

