Metabolic pathways and immunometabolism in rare kidney diseases
- PMID: 29724730
- PMCID: PMC6045442
- DOI: 10.1136/annrheumdis-2017-212935
Metabolic pathways and immunometabolism in rare kidney diseases
Abstract
Objectives: To characterise renal tissue metabolic pathway gene expression in different forms of glomerulonephritis.
Methods: Patients with nephrotic syndrome (NS), antineutrophil cytoplasmic antibody-associated vasculitis (AAV), systemic lupus erythematosus (SLE) and healthy living donors (LD) were studied. Clinically indicated renal biopsies were obtained at time of diagnosis and microdissected into glomerular and tubulointerstitial compartments. Microarray-derived differential gene expression of 88 genes representing critical enzymes of metabolic pathways and 25 genes related to immune cell markers was compared between disease groups. Correlation analyses measured relationships between metabolic pathways, kidney function and cytokine production.
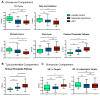
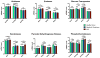
Results: Reduced steady state levels of mRNA species were enriched in pathways of oxidative phosphorylation and increased in the pentose phosphate pathway (PPP) with maximal perturbation in AAV and SLE followed by NS, and least in LD. Transcript regulation was isozymes specific with robust regulation in hexokinases, enolases and glucose transporters. Intercorrelation networks were observed between enzymes of the PPP (eg, transketolase) and macrophage markers (eg, CD68) (r=0.49, p<0.01). Increased PPP transcript levels were associated with reduced glomerular filtration rate in the glomerular (r=-0.49, p<0.01) and tubulointerstitial (r=-0.41, p<0.01) compartments. PPP expression and tumour necrosis factor activation were tightly co-expressed (r=0.70, p<0.01).
Conclusion: This study demonstrated concordant alterations of the renal transcriptome consistent with metabolic reprogramming across different forms of glomerulonephritis. Activation of the PPP was tightly linked with intrarenal macrophage marker expression, reduced kidney function and increased production of cytokines. Modulation of glucose metabolism may offer novel immune-modulatory therapeutic approaches in rare kidney diseases.
Keywords: granulomatosis with polyangiitis; lupus nephritis; systemic vasculitis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

