Establishing the Transient Mass Balance of Thrombosis: From Tissue Factor to Thrombin to Fibrin Under Venous Flow
- PMID: 29724819
- PMCID: PMC6023760
- DOI: 10.1161/ATVBAHA.118.310906
Establishing the Transient Mass Balance of Thrombosis: From Tissue Factor to Thrombin to Fibrin Under Venous Flow
Abstract
Objective: We investigated the coregulation of thrombin and fibrin as blood flows over a procoagulant surface.
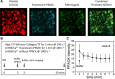
Approach and results: Using microfluidic perfusion of factor XIIa-inhibited human whole blood (200 s-1 wall shear rate) over a 250-μm long patch of collagen/TF (tissue factor; ≈1 molecule per μm2) and immunoassays of the effluent for F1.2 (prothrombin fragment 1.2), TAT (thrombin-antithrombin complex), and D-dimer (post-end point plasmin digest), we sought to establish the transient mass balance for clotting under venous flow. F1.2 (but almost no free thrombin detected via TAT assay) continually eluted from clots when fibrin was allowed to form. Low-dose fluorescein-Phe-Pro-Arg-chloromethylketone stained fibrin-bound thrombin-a staining ablated by anti-γ'-fibrinogen or the fibrin inhibitor glypro-arg-pro but highly resistant to 7-minute buffer rinse, demonstrating tight binding of thrombin to γ'-fibrin. With fibrin polymerizing for 500 seconds, 92 000 thrombin molecules and 203 000 clot-associated fibrin monomer equivalents were generated per TF molecule (or per μm2). Fibrin reached 15 mg/mL in the pore space (porosity ≈0.5) of a 15-μm-thick thrombus core by 500 seconds and 30 mg/mL by 800 seconds. For a known rate of ≈60 FPA (fibrinopeptide-A) per thrombin per second, each thrombin molecule generated only 3 fibrin monomer equivalents during 500 seconds, indicating an intraclot thrombin half-life of ≈70 ms, much shorter than its diffusional escape time (≈10 seconds). By 800 seconds, gly-pro-arg-pro allowed 4-fold more F1.2 generation, consistent with gly-pro-arg-pro ablating fibrin's antithrombin-I activity and facilitating thrombin-mediated FXIa activation.
Conclusions: Under flow, fibrinogen continually penetrates the clot, and γ'-fibrin regulates thrombin.
Keywords: blood; fibrin; hemodynamics; hemostasis; platelets; thrombosis.
© 2018 American Heart Association, Inc.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

