Gut microbiota utilize immunoglobulin A for mucosal colonization
- PMID: 29724905
- PMCID: PMC5973787
- DOI: 10.1126/science.aaq0926
Gut microbiota utilize immunoglobulin A for mucosal colonization
Abstract
The immune system responds vigorously to microbial infection while permitting lifelong colonization by the microbiome. Mechanisms that facilitate the establishment and stability of the gut microbiota remain poorly described. We found that a regulatory system in the prominent human commensal Bacteroides fragilis modulates its surface architecture to invite binding of immunoglobulin A (IgA) in mice. Specific immune recognition facilitated bacterial adherence to cultured intestinal epithelial cells and intimate association with the gut mucosal surface in vivo. The IgA response was required for B. fragilis (and other commensal species) to occupy a defined mucosal niche that mediates stable colonization of the gut through exclusion of exogenous competitors. Therefore, in addition to its role in pathogen clearance, we propose that IgA responses can be co-opted by the microbiome to engender robust host-microbial symbiosis.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
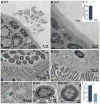

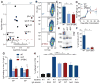
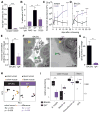
Comment in
-
Unlocking the secrets of IgA.Nat Rev Gastroenterol Hepatol. 2018 Jul;15(7):389. doi: 10.1038/s41575-018-0036-3. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29849107 No abstract available.
-
Gut Microbiota: IgA Protects the Pioneers.Curr Biol. 2018 Sep 24;28(18):R1117-R1119. doi: 10.1016/j.cub.2018.08.019. Curr Biol. 2018. PMID: 30253156
References
-
- Fagarasan S, et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

