Glycine N-methyltransferase inhibits aristolochic acid nephropathy by increasing CYP3A44 and decreasing NQO1 expression in female mouse hepatocytes
- PMID: 29725048
- PMCID: PMC5934382
- DOI: 10.1038/s41598-018-22298-6
Glycine N-methyltransferase inhibits aristolochic acid nephropathy by increasing CYP3A44 and decreasing NQO1 expression in female mouse hepatocytes
Abstract
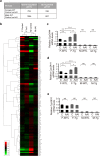
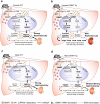
Plants containing aristolochic acids (AA) are nephrotoxins. Glycine N-methyltransferase (GNMT) acts to bind environmental toxins such as benzo(a)pyrene and aflatoxin B1, translocate into nucleus, and alter hepatic metabolism. This study aims to determine the role of GNMT in AA-induced nephropathy. We established an AA nephropathy mouse model and found that AA type I (AAI)-induced nephropathy at a lower concentration in male than in female mice, implying sex differences in AAI resistance. Microarray analysis and AAI-treated mouse models showed that GNMT moderately reduced AAI-induced nephropathy by lowering the upregulated level of NQO1 in male, but significantly improved the nephropathy additionally by increasing Cyp3A44/3A41 in female. The protective effects of GNMT were absent in female GNMT knockout mice, in which re-expression of hepatic GNMT significantly decreased AAI-induced nephropathy. Mechanism-wise, AAI enhanced GNMT nuclear translocation, resulting in GNMT interaction with the promoter region of the genes encoding Nrf2 and CAR/PXR, the transcription factors for NQO1 and CYP3A44/3A41, respectively. Unlike the preference for Nrf2/NQO1 transcriptions at lower levels of GNMT, overexpression of GNMT preferred CAR/PXR/CYP3A44/3A41 transcriptions and alleviated kidney injury upon AAI treatment. In summary, hepatic GNMT protected mice from AAI nephropathy by enhancing CAR/PXR/CYP3A44/3A41 transcriptions and reducing Nrf2/NQO1 transcriptions.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- IARC Working Group W. A review of human carcinogens. Part A: Pharmaceuticals. Plants containing aristolochic acid.. IARC Monogr. Eval. Carcinog. Risks Hum. 2012;100A:347–361.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

