Fine Localization of Acetylcholinesterase in the Synaptic Cleft of the Vertebrate Neuromuscular Junction
- PMID: 29725289
- PMCID: PMC5917012
- DOI: 10.3389/fnmol.2018.00123
Fine Localization of Acetylcholinesterase in the Synaptic Cleft of the Vertebrate Neuromuscular Junction
Abstract
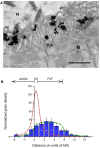
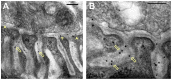
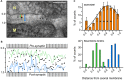
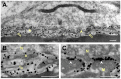
Acetylcholinesterase (AChE) is concentrated at cholinergic synapses, where it is a major factor in controlling the duration of transmitter action. The concentration and localization of AChE within the synaptic cleft are in keeping with the functional requirements of the particular type of synapse. The densities of synaptic AChE at various neuromuscular junctions (NMJs) had been evaluated by quantitative EM-autoradiography using radiolabeled probes. Yet, fundamental issues concerning the precise distribution and location of the enzyme in the cleft remained open: whether and to what extent synaptic AChE is associated with pre- or postsynaptic membranes, or with synaptic basal lamina (BL), and whether it occurs only in the primary cleft (PC) or also in postjunctional folds (PJFs). Nanogold-conjugates of fasciculin, an anticholinesterase polypeptide toxin, were prepared and used to label AChE at NMJs of mouse and frog muscles. Selective intense labeling was obtained at the NMJs, with gold-labeled AChE sites distributed over the BL in the PC and the PJFs. Quantitative analysis demonstrated that AChE sites are almost exclusively located on the BL rather than on pre- or postsynaptic membranes and are distributed in the PC and down the PJFs, with a defined pattern. This localization pattern of AChE is suggested to ensure full hydrolysis of acetylcholine (ACh) bouncing off receptors, thus eliminating its unnecessary detrimental reattachment.
Keywords: acetylcholinesterase; basal lamina; nanogold; postjunctional folds; synaptic cleft.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

