Automated Morphological Analysis of Microglia After Stroke
- PMID: 29725290
- PMCID: PMC5917008
- DOI: 10.3389/fncel.2018.00106
Automated Morphological Analysis of Microglia After Stroke
Abstract
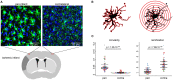
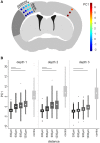
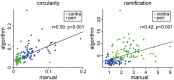
Microglia are the resident immune cells of the brain and react quickly to changes in their environment with transcriptional regulation and morphological changes. Brain tissue injury such as ischemic stroke induces a local inflammatory response encompassing microglial activation. The change in activation status of a microglia is reflected in its gradual morphological transformation from a highly ramified into a less ramified or amoeboid cell shape. For this reason, the morphological changes of microglia are widely utilized to quantify microglial activation and studying their involvement in virtually all brain diseases. However, the currently available methods, which are mainly based on manual rating of immunofluorescent microscopic images, are often inaccurate, rater biased, and highly time consuming. To address these issues, we created a fully automated image analysis tool, which enables the analysis of microglia morphology from a confocal Z-stack and providing up to 59 morphological features. We developed the algorithm on an exploratory dataset of microglial cells from a stroke mouse model and validated the findings on an independent data set. In both datasets, we could demonstrate the ability of the algorithm to sensitively discriminate between the microglia morphology in the peri-infarct and the contralateral, unaffected cortex. Dimensionality reduction by principal component analysis allowed to generate a highly sensitive compound score for microglial shape analysis. Finally, we tested for concordance of results between the novel automated analysis tool and the conventional manual analysis and found a high degree of correlation. In conclusion, our novel method for the fully automatized analysis of microglia morphology shows excellent accuracy and time efficacy compared to traditional analysis methods. This tool, which we make openly available, could find application to study microglia morphology using fluorescence imaging in a wide range of brain disease models.
Keywords: image analysis; microglia; morphology; neuroinflammation; stroke.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

