Comparing Reentrant Drivers Predicted by Image-Based Computational Modeling and Mapped by Electrocardiographic Imaging in Persistent Atrial Fibrillation
- PMID: 29725307
- PMCID: PMC5917348
- DOI: 10.3389/fphys.2018.00414
Comparing Reentrant Drivers Predicted by Image-Based Computational Modeling and Mapped by Electrocardiographic Imaging in Persistent Atrial Fibrillation
Abstract
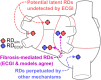
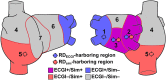
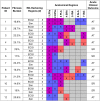
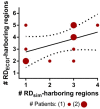
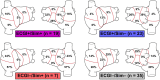
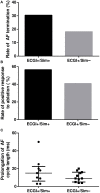
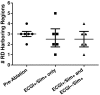
Electrocardiographic mapping (ECGI) detects reentrant drivers (RDs) that perpetuate arrhythmia in persistent AF (PsAF). Patient-specific computational models derived from late gadolinium-enhanced magnetic resonance imaging (LGE-MRI) identify all latent sites in the fibrotic substrate that could potentially sustain RDs, not just those manifested during mapped AF. The objective of this study was to compare RDs from simulations and ECGI (RDsim/RDECGI) and analyze implications for ablation. We considered 12 PsAF patients who underwent RDECGI ablation. For the same cohort, we simulated AF and identified RDsim sites in patient-specific models with geometry and fibrosis distribution from pre-ablation LGE-MRI. RDsim- and RDECGI-harboring regions were compared, and the extent of agreement between macroscopic locations of RDs identified by simulations and ECGI was assessed. Effects of ablating RDECGI/RDsim were analyzed. RDsim were predicted in 28 atrial regions (median [inter-quartile range (IQR)] = 3.0 [1.0; 3.0] per model). ECGI detected 42 RDECGI-harboring regions (4.0 [2.0; 5.0] per patient). The number of regions with RDsim and RDECGI per individual was not significantly correlated (R = 0.46, P = ns). The overall rate of regional agreement was fair (modified Cohen's κ0 statistic = 0.11), as expected, based on the different mechanistic underpinning of RDsim- and RDECGI. nineteen regions were found to harbor both RDsim and RDECGI, suggesting that a subset of clinically observed RDs was fibrosis-mediated. The most frequent source of differences (23/32 regions) between the two modalities was the presence of RDECGI perpetuated by mechanisms other than the fibrotic substrate. In 6/12 patients, there was at least one region where a latent RD was observed in simulations but was not manifested during clinical mapping. Ablation of fibrosis-mediated RDECGI (i.e., targets in regions that also harbored RDsim) trended toward a higher rate of positive response compared to ablation of other RDECGI targets (57 vs. 41%, P = ns). Our analysis suggests that RDs in human PsAF are at least partially fibrosis-mediated. Substrate-based ablation combining simulations with ECGI could improve outcomes.
Keywords: ablation; atrial fibrillation; computational modeling; electrocardiographic mapping; fibrotic remodeling; reentrant drivers.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

