Low-dose aspirin at ≤16 weeks of gestation for preventing preeclampsia and its maternal and neonatal adverse outcomes: A systematic review and meta-analysis
- PMID: 29725376
- PMCID: PMC5920352
- DOI: 10.3892/etm.2018.5972
Low-dose aspirin at ≤16 weeks of gestation for preventing preeclampsia and its maternal and neonatal adverse outcomes: A systematic review and meta-analysis
Abstract
The aim of the present meta-analysis study was to evaluate the efficacy of low-dose aspirin, commenced at ≤16 weeks of gestation, in preventing preterm and term preeclampsia, as well as associated maternal and neonatal adverse events in women at risk of preeclampsia. The Embase, PubMed, Cochrane Central Register of Controlled Trials and the Web of Science databases were searched for relevant random controlled trials (RCTs) published between January 1979 and October 2017. After quality assessment and data extraction, a meta-analysis was performed using RevMan 5.3 software. Outcomes of interest were preeclampsia with subgroups of preterm preeclampsia (delivery at <37 weeks) and term preeclampsia, as well as maternal adverse outcomes, including gestational hypertension, postpartum hemorrhage and preterm birth, and neonatal adverse outcomes, including intrauterine growth retardation (IUGR) or small for gestation age infant (SGA), stillbirth or death, and newborn weight. A total of 10 RCTs involving 3,168 participants were included. The meta-analysis demonstrated that, compared with placebo or no treatment, low-dose aspirin was associated with a significant reduction in the overall risk ratio (RR) of preeclampsia regardless of the time to delivery [RR=0.67; 95% confidence interval (CI)=0.57-0.80]. This was apparent for preterm preeclampsia (RR=0.35; 95% CI=0.13-0.94) but not for term preeclampsia (RR=1.01; 95% CI=0.60-1.70). Except for postpartum hemorrhage, low-dose aspirin also significantly reduced the risk of maternal and neonatal adverse outcomes. In conclusion, low-dose aspirin in women at risk of preeclampsia, commenced at ≤16 weeks of gestation, was associated with a reduced risk of preterm preeclampsia, and of adverse maternal and neonatal outcomes.
Keywords: low-dose aspirin; meta-analysis; preeclampsia.
Figures
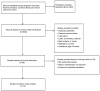
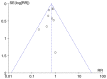
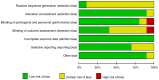


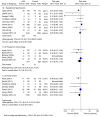

References
-
- Groeneveld E, Lambers MJ, Lambalk CB, Broeze KA, Haapsamo M, de Sutter P, Schoot BC, Schats R, Mol BW, Hompes PG. Preconceptional low-dose aspirin for the prevention of hypertensive pregnancy complications and preterm delivery after IVF: A meta-analysis with individual patient data. Hum Reprod. 2013;28:1480–1488. doi: 10.1093/humrep/det022. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
