Exogenous hydrogen sulfide promotes hepatocellular carcinoma cell growth by activating the STAT3-COX-2 signaling pathway
- PMID: 29725404
- PMCID: PMC5920354
- DOI: 10.3892/ol.2018.8154
Exogenous hydrogen sulfide promotes hepatocellular carcinoma cell growth by activating the STAT3-COX-2 signaling pathway
Abstract
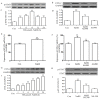
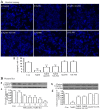
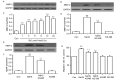
The effects of hydrogen sulfide (H2S) on cancer are controversial. Our group previously demonstrated that exogenous H2S promotes the development of cancer via amplifying the activation of the nuclear factor-κB signaling pathway in hepatocellular carcinoma (HCC) cells (PLC/PRF/5). The present study aimed to further investigate the hypothesis that exogenous H2S promotes PLC/PRF/5 cell proliferation and migration, and inhibits apoptosis by activating the signal transducer and activator of transcription 3 (STAT3)-cyclooxygenase-2 (COX-2) signaling pathway. PLC/PRF/5 cells were treated with 500 µmol/l NaHS (a donor of H2S) for 24 h. The expression levels of phosphorylated (p)-STAT3, STAT3, cleaved caspase-3 and COX-2 were measured by western blot assay. Cell viability was detected by Cell Counting kit-8 assay. Apoptotic cells were observed by Hoechst 33258 staining. The expression of STAT3 and COX-2 messenger RNA (mRNA) was detected by semiquantitative reverse transcription-polymerase chain reaction. The production of vascular endothelial growth factor (VEGF) was evaluated by ELISA. The results indicated that treatment of PLC/PRF/5 cells with 500 µmol/l NaHS for 24 h markedly increased the expression levels of p-STAT3 and STAT3 mRNA, leading to COX-2 and COX-2 mRNA overexpression, VEGF induction, decreased cleaved caspase-3 production, increased cell viability and migration, and decreased number of apoptotic cells. However, co-treatment of PLC/PRF/5 cells with 500 µmol/l NaHS and 30 µmol/l AG490 (an inhibitor of STAT3) or 20 µmol/l NS-398 (an inhibitor of COX-2) for 24 h significantly reverted the effects induced by NaHS. Furthermore, co-treatment of PLC/PRF/5 cells with 500 µmol/l NaHS and 30 µmol/l AG490 markedly decreased the NaHS-induced increase in the expression level of COX-2. By contrast, co-treatment of PLC/PRF/5 cells with 500 µmol/l NaHS and 20 µmol/l NS-398 inhibited the NaHS-induced increase in the expression level of p-STAT3. In conclusion, the findings of the present study provide evidence that the STAT3-COX-2 signaling pathway is involved in NaHS-induced cell proliferation, migration, angiogenesis and anti-apoptosis in PLC/PRF/5 cells, and suggest that the positive feedback between STAT3 and COX-2 may serve a crucial role in hepatocellular carcinoma carcinogenesis.
Keywords: COX-2; PLC/PRF/5 cells; STAT3; growth; hydrogen sulfide.
Figures


References
-
- Verslype C, Van Cutsem E, Dicato M, Arber N, Berlin JD, Cunningham D, De Gramont A, Diaz-Rubio E, Ducreux M, Gruenberger T, et al. The management of hepatocellular carcinoma. current expert opinion and recommendations derived from the 10th World Congress on Gastrointestinal Cancer, Barcelona, 2008. Ann Oncol. 2009;20(Suppl 7):Svii1–Svii6. doi: 10.1093/annonc/mdp281. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
