The untold stories of the speech gene, the FOXP2 cancer gene
- PMID: 29725501
- PMCID: PMC5931254
- DOI: 10.18632/genesandcancer.169
The untold stories of the speech gene, the FOXP2 cancer gene
Abstract
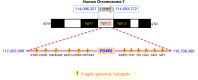
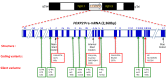
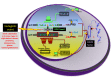
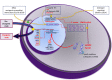
FOXP2 encodes a transcription factor involved in speech and language acquisition. Growing evidence now suggests that dysregulated FOXP2 activity may also be instrumental in human oncogenesis, along the lines of other cardinal developmental transcription factors such as DLX5 and DLX6 [1-4]. Several FOXP familymembers are directly involved during cancer initiation, maintenance and progression in the adult [5-8]. This may comprise either a pro-oncogenic activity or a deficient tumor-suppressor role, depending upon cell types and associated signaling pathways. While FOXP2 is expressed in numerous cell types, its expression has been found to be down-regulated in breast cancer [9], hepatocellular carcinoma [8] and gastric cancer biopsies [10]. Conversely, overexpressed FOXP2 has been reported in multiple myelomas, MGUS (Monoclonal Gammopathy of Undetermined Significance), several subtypes of lymphomas [5,11], as well as in neuroblastomas [12] and ERG fusion-negative prostate cancers [13]. According to functional evidences reported in breast cancer [9] and survey of recent transcriptomic and proteomic analyses of different tumor biopsies, we postulate that FOXP2 dysregulation may play a main role throughout cancer initiation and progression. In some cancer conditions, FOXP2 levels are now considered as a critical diagnostic marker of neoplastic cells, and in many situations, they even bear strong prognostic value [5]. Whether FOXP2 may further become a therapeutic target is an actively explored lead. Knowledge reviewed here may help improve our understanding of FOXP2 roles during oncogenesis and provide cues for diagnostic, prognostic and therapeutic analyses.
Keywords: FOXP2 factor; cancer; language; oncogene; prognosis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


References
-
- Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–85. - PubMed
-
- Gitton Y, Levi G. DLX2 (Distal-less Homeobox 2) Atlas Genet Cytogenet Oncol Haematol. 2014;18:805–9.
-
- Gitton Y, Levi G. DLX5 (Distal-less Homeobox 5) Atlas Genet Cytogenet Oncol Haematol. 2014;18:810–6.
-
- Gitton Y, Levi G. DLX6 (Distal-less Homeobox 6) Atlas Genet Cytogenet Oncol Haematol. 2014;18:817–23.
-
- Campbell AJ, Lyne L, Brown PJ, Launchbury RJ, Bignone P, Chi J, Roncador G, Lawrie CH, Gatter KC, Kusec R, Banham AH. Aberrant expression of the neuronal transcription factor FOXP2 in neoplastic plasma cells. Br J Haematol. 2010;149:221–30. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

