Organizing biochemistry in space and time using prion-like self-assembly
- PMID: 29725624
- PMCID: PMC5926789
- DOI: 10.1016/j.coisb.2017.11.012
Organizing biochemistry in space and time using prion-like self-assembly
Abstract
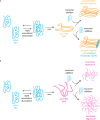
Prion-like proteins have the capacity to adopt multiple stable conformations, at least one of which can recruit proteins from the native conformation into the alternative fold. Although classically associated with disease, prion-like assembly has recently been proposed to organize a range of normal biochemical processes in space and time. Organisms from bacteria to mammals use prion-like mechanisms to (re)organize their proteome in response to intracellular and extracellular stimuli. Prion-like behavior is an economical means to control biochemistry and gene regulation at the systems level, and prions can act as protein-based genes to facilitate quasi-Lamarckian inheritance of induced traits. These mechanisms allow individual cells to express distinct heritable traits using the same complement of polypeptides. Understanding and controlling prion-like behavior is therefore a promising strategy to combat diverse pathologies and organize engineered biological systems.
Figures

References
-
- Cajal S. Un sencillo metodo de coloracion selectiva del reticulo protoplasmico y sus efectos en los diversos organos nerviosos de vertebrados e invertebrados. Trab Lab Investig Biol Univ Madr. 1903;2:129–221.
-
- van Leeuwenhoek A. Observations On Animalcula Seen In Rain, Well, Sea And Snow-Water; As Also In Pepper-Water. :1675.
-
- Patino MM, Liu J-J, Glover JR, Lindquist S. Support for the Prion Hypothesis for Inheritance of a Phenotypic Trait in Yeast. Science. 1996;273:622–626. This manuscript convincingly demonstrates that the [PSI+] state of S. cerevisiae is conferred by a prion form of the Sup35 protein. - PubMed
-
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. Prusiner shows that the causative agent of scrapie, long observed to have unusual properties, is caused by an infectious protein, or prion. - PubMed
-
- Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. In this foundational study, Wickner shows that the [URE3+] state in S. cerevisiae is conferred by a prion form of the Ure2 protein, and speculates that [PSI+] might likewise be prion-like in nature. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
